实验性诱导营养不良对鸡脑部抑郁和情绪中等以及血液影响的生物化学作用
DOI:
https://doi.org/10.18686/zhfnc.v1i2.71关键词:
鸡;营养不良;脑功能;血液指数;贫血;CBC摘要
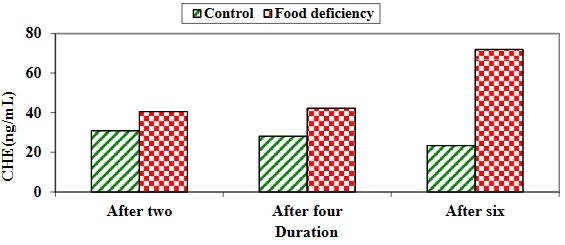
背景:营养缺乏和营养不良会引起周期性嗜睡、情绪低落和贫血。因此,在本研究中,我们通过实验诱导鸡营养不良,以跟踪鸡的脑功能和贫血状况(血液指数)。材料和方法:本研究以两组60只一天龄的雏鸡为研究对象,这些雏鸡喂食商品开食料10天,然后平均分成两组,饲养6周。第一对照组在生长期和育成期饲喂商品生长料和育成料,而第二试验组在生长期和育成期持续饲喂黄玉米。所有雏鸡在六周内每周称重一次,以记录体重差异。每两周从每组的10只雏鸡中采集全血样和脑匀浆,以评估脑组织匀浆参数、白细胞计数差异、全血细胞计数和血液指数。结果:比较两组雏鸡的脑组织匀浆发现,缺食组的乙酰胆碱酯酶(AChE)和总抗氧化能力(TAC)在整个饲养过程中显著增加,而肿瘤坏死因子-α(TNF-α)在缺食组第二周和第四周后增加,第六周后两组无显著差异。对照组的谷胱甘肽过氧化物酶(GPx)和超氧化物歧化酶(SOD)在整个实验期间都明显增加,而全血细胞计数(CBC)和细胞差异计数显示,食物缺乏组的白细胞(WBCs)在整个实验期间都明显增加、嗜酸性粒细胞浓度在缺食组两周和四周后明显升高,六周后两组差异不明显;淋巴细胞在对照组第二周后明显升高,四周后差异不明显,六周后缺食组则明显升高。缺食组的中性粒细胞计数在第六周后明显偏高,单核细胞浓度在缺食组中两周后明显升高,四周后无明显差异,在对照组中六周后明显升高。嗜碱性粒细胞和分段中性粒细胞在食物缺乏组两周后明显增加,两组在四周和六周后差异不明显。此外,对照组的血红蛋白(Hb)、红细胞(RBCs)、包装细胞容积(PCV)、平均体细胞容积(MCV)和血小板计数在整个持续时间内均显著增加,对照组的平均体细胞血红蛋白(MCH)和平均体细胞血红蛋白浓度(MCHC)在四周和六周后显著增加。结论:营养不良会对大脑功能和血液参数产生很大影响。
##submission.downloads##
已出版
文章引用
期
栏目
执照
版权声明
CC BY-NC 4.0作者应保留其作品的版权,并授予期刊/出版商首次出版该作品的权利,同时根据以下条款获得许可: 知识共享署名-非商业性4.0国际版(CC BY-NC 4.0)。本许可证允许复制、分发和传播作品,前提是声明了原创作者的正确归属。
参考
1. Getawa S, Getaneh Z, Melku M. Hematological abnormalities and associated factors among undernourished under-five children attending University of Gondar Specialized Referral Hospital, northwest Ethiopia. Journal of Blood Medicine 2020; 11: 465–478. doi: 10.2147/JBM.S284572
2. Espinoza M, Perelli J, Olmos R, et al. Nutritional assessment as predictor of complications after hematopoietic stem cell transplantation. Revista Brasileira de Hematologia e Hemoterapia 2016; 38(1): 7–14. doi: 10.1016/j.bjhh.2015.10.002
3. Mansour HM. Nutrition and brain functions in health and disease. In: Mohamed W, Kobeissy F (editors). Nutrition and Psychiatric Disorders. Springer; 2022. pp. 3–26.
4. Favela LH, Martin J. “Cognition” and dynamical cognitive science. Minds and Machines 2017; 27(2): 331–355. doi: 10.1007/s11023-016-9411-4
5. Okon-Singer H, Hendler T, Pessoa L, Shackman AJ. The neurobiology of emotion-cognition interactions: Fundamental questions and strategies for future research. Frontiers in Human Neuroscience 2015; 9: 58. doi: 10.3389/fnhum.2015.00058
6. Galbiati A, d’Adda di Fagagna F. DNA damage in situ ligation followed by proximity ligation assay (DI-PLA). Methods in Molecular Biology 2019; 1896: 13–20. doi: 10.1007/978-1-4939-8931-7_2
7. Ellman GL, Courtney KD, Andres V Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology 1961; 7(2): 88–95. doi: 10.1016/0006-2952(61)90145-9
8. Azhar S, Cao L, Reaven E. Alteration of the adrenal antioxidant defense system during aging in rats. The Journal of Clinical Investigation 1995; 96(3): 1414–1424. doi: 10.1172/JCI118177
9. Petrovas C, Daskas SM, Lianidou ES. Determination of tumor necrosis factor-alpha (TNF-alpha) in serum by a highly sensitive enzyme amplified lanthanide luminescence immunoassay. Clinical Biochemistry 1999; 32(4): 241–247. doi: 10.1016/s0009-9120(99)00004-1
10. Nebot C, Moutet M, Huet P, et al. Spectrophotometric Assay of SOD activity based on the activated auto-oxidation of tetracyclic catechol. Analytical Biochemistry 1993; 214(2): 442–451. doi: 10.1006/abio.1993.1521
11. Nelly A, J.Randall S, Ivan SP, Harvey JC. Partial sequences of human plasma glutathione peroxidase and immunologic identification of milk glutathione peroxidase as the plasma enzyme. The Journal of Nutrition 1991; 121(8): 1243–1249. doi: 10.1093/jn/121.8.1243
12. Steel RGD, Torrie JH, Dickey DA. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed. McGraw-Hill; 1997.
13. Zielińska M, Łuszczki E, Dereń K. Dietary nutrient deficiencies and risk of depression (Review article 2018–2023). Nutrients 2023; 15(11): 2433. doi: 10.3390/nu15112433
14. Huang Q, Liu H, Suzuki K, et al. Linking What we eat to our mood: A review of diet, dietary antioxidants, and depression. Antioxidants 2019; 8(9): 376. doi: 10.3390/antiox8090376
15. Winick M, Noble A. Cellular response in rats during malnutrition at various ages. The Journal of Nutrition 1966; 89(3): 300–306. doi: 10.1093/jn/89.3.300
16. Crnic LS. Effects of nutrition and environment on brain biochemistry and behavior. Development Psychobiology 1983; 16(2): 129–145. doi: 10.1002/dev.420160206
17. Zivkovic AR, Paul GM, Hofer S, et al. Increased enzymatic activity of acetylcholinesterase indicates the severity of the sterile inflammation and predicts patient outcome following traumatic injury. Biomolecules 2023; 13(2): 267. doi: 10.3390/biom13020267
18. Nizri E, Hamra‐Amitay Y, Sicsic C, et al. Anti‐inflammatory properties of cholinergic up‐regulation: A new role for acetylcholinesterase inhibitors. Neuropharmacology 2006; 50(5): 540–547. doi: 10.1016/j.neuropharm.2005.10.013
19. Rosas‐Ballina M, Olofsson PS, Ochani M, et al. Acetylcholine‐synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011; 334(6052): 98–101. doi: 10.1126/science.1209985
20. Ben‐David Y, Kagan S, Cohen Ben‐Ami H, et al. RIC3, the cholinergic anti‐inflammatory pathway, and neuroinflammation. International Immunopharmacol 2020; 83: 106381. doi: 10.1016/j.intimp.2020.106381
21. Prenesti E, Berto S, Gosmaro F, et al. Dysmetabolisms can affect total antioxidant capacity (TAC) of human plasma: Determination of reference intervals of TAC by way of CUPRAC-BCS method. Antioxidants 2021; 10(1): 58. doi: 10.3390/antiox10010058
22. Feoli AM, Siqueira IR, Almeida L, et al. Effects of protein malnutrition on oxidative status in rat brain. Nutrition 2006; 22(2): 160–165. doi: 10.1016/j.nut.2005.06.007
23. Azevedo ZM, Luz RA, Victal SH, et al. Increased production of tumor necrosis factor-alpha in whole blood cultures from children with primary malnutrition. Brazilian Journal of Medical and Biological Research 2005; 38(2): 171–183. doi: 10.1590/s0100-879x2005000200005
24. Feuerstein GZ, Liu T, Barone FC. Cytokines, inflammation, and brain injury: Role of tumor necrosis factor-alpha. Cerebrovascular and Brain Metabolism Reviews 1994; 6(4): 341–360.
25. Arvin B, Neville LF, Barone FC, Feuerstein GZ. The role of inflammation and cytokines in brain injury. Neuroscience & Biobehavioral Reviews 1996; 20(3): 445–452. doi: 10.1016/0149-7634(95)00026-7
26. Kochanek PM, Hallenbeck JM. Polymorphonuclear leukocytes and monocytes/macrophages in the pathogenesis of cerebral ischemia and stroke. Stroke 1992; 23: 1367–1379. doi: 10.1161/01.STR.23.9.1367
27. Postal M, Lapa AT, Sinicato NA, et al. Depressive symptoms are associated with tumor necrosis factor alpha in systemic lupus erythematosus. Journal of Neuroinflammation 2016; 13(5). doi: 10.1186/s12974-015-0471-9
28. Himmerich H. Activity of the TNF-α system in patients with brain disorders and during psychopharmacological treatment. Current Pharmaceutical Analysis 2007; 3(1): 1–5. doi: 10.2174/157341207779802412
29. Matés JM, Pérez-Gómez C, Núñez de Castro I, et al. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. The International Journal of Biochemistry & Cell Biology 2002; 34(5): 439–458. doi: 10.1016/s1357-2725(01)00143-1
30. Olalla L, Aledo JC, Bannenberg G, Márquez J. The C-terminus of human glutaminase L mediates association with PDZ domain-containing proteins. FEBS Letters 2001; 488(3): 116–122. doi: 10.1016/s0014-5793(00)02373-5
31. Halliwell B. Oxygen radicals as key mediators in neurological disease: Fact or fiction? Annals of Neurology 1992; 32(S1): S10–S15. doi: 10.1002/ana.410320704
32. Arya AK, Kumar P, Midha Tanu, Singh M. Hematological profile of children with severe acute malnutrition: A tertiary care centre experience. International Journal of Contemporary Pediatrics 2017; 4(5): 1577–1580. doi: 10.18203/2349-3291.ijcp20173072
33. Khan S, Rubab Z, Hussain S, et al. Hematological profile of children with severe acute malnutrition at the Tertiary care hospital in Multan. Isra Medical Journal 2020; 12(1): 12–16.
34. Gohain EK, Pathak K, Choudhury B. A case control study of hematological changes in children with protein energy malnutrition attending Gauhati medical college and hospital. IOSR Journal of Dental and Medical Sciences 2016; 15(10): 25–29. doi: 10.9790/0853-1510012529
35. Jordan S, Tung N, Casanova-Acebes M, et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell 2019; 178(5): 1102–1114. doi: 10.1016/j.cell.2019.07.050
36. Corware K, Yardley V, Mack C, et al. Protein energy malnutrition increases arginase activity in monocytes and macrophages. Nutrition & Metabolism 2014; 11(51). doi: 10.1186/1743-7075-11-51
37. Takele Y, Adem E, Getahun M, et al. Malnutrition in healthy individuals results in increased mixed cytokine profiles, altered neutrophil subsets and function. PLoS One 2016; 11(8): e0157919. doi: 10.1371/journal.pone.0157919
38. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. The Journal of Gerontology: Series A 2014; 69(Suppl 1): S4–S9. doi: 10.1093/gerona/glu057
39. Tigner A, Ibrahim SA, Murray IV. Histology, white blood cell. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563148/ (accessed on 26 September 2023).


