Surface dependent effects of Mn-doping on the catalytic activity of α-Fe2O3 for ortho-para hydrogen conversion
DOI:
https://doi.org/10.18686/cest574Keywords:
hydrogen liquefaction; ortho-para hydrogen conversion; iron oxide; surface dopingAbstract
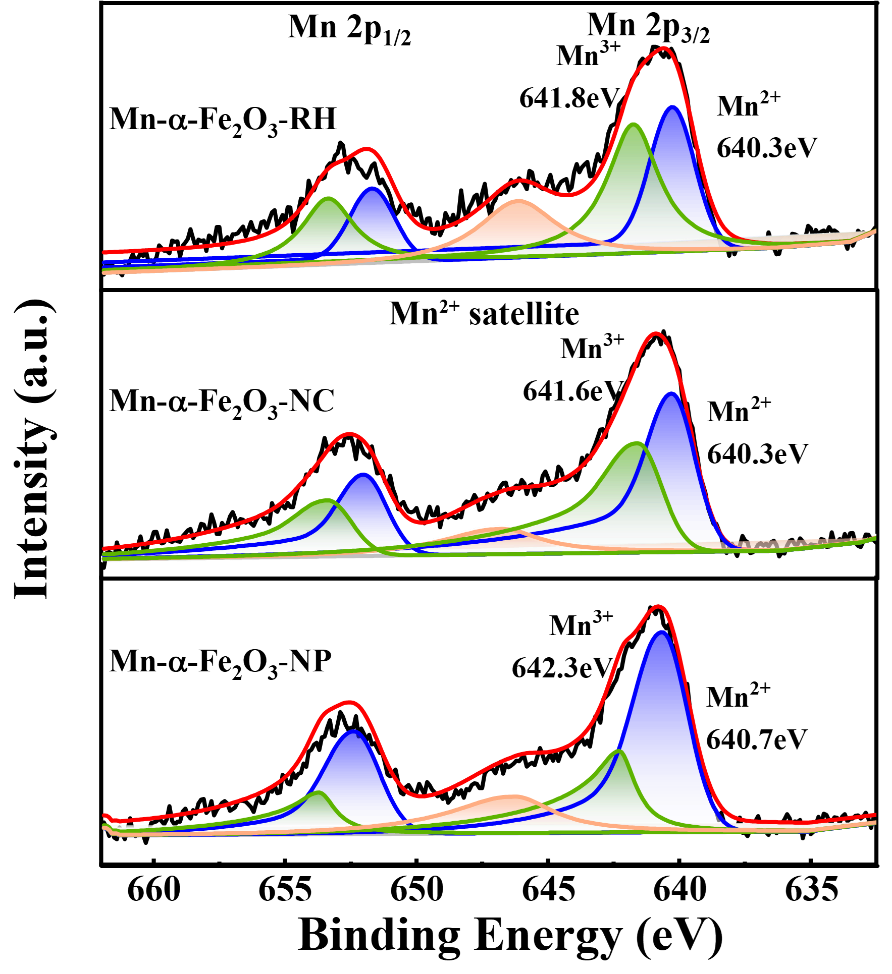
Ortho-para hydrogen conversion is a crucial step in the large-scale production, storage, and transportation of liquid hydrogen. Iron-based oxides are widely used catalysts for ortho-para hydrogen conversion, with transition metal doping serving as the most promising method to enhance their catalytic activity. In this work, we investigated the effect of Mn doping on ortho-para hydrogen conversion over α-Fe2O3 catalysts with different morphologies. It was found that Mn-doping effect on ortho-para hydrogen conversion was morphology-dependent, i.e., Mn doping increased activity in cubic and rhombohedral samples but decreased it in nanosheet samples. Combined characterizations indicate that the activity loss in Mn-α-Fe2O3-NP arises from reduced magnetization caused by a lower surface content of Mn3+ on α-Fe2O3(001) surface, whereas the activity increases in Mn-α-Fe2O3-NC and Mn-α-Fe2O3-RH correlate with a larger proportion of high valance Mn3+ and a slight increase in magnetization over (012) and (104) facets, demonstrating that the effect of metal doping depends strongly on surface facets which governs the surface structure and magnetic properties of catalysts.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Author(s)

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
1. Bhuiyan MMH, Sakib AN, Alawee SI, et al. Fueling the future: A comprehensive analysis and forecast of fuel consumption trends in US electricity generation. Sustainability, 2024, 16(6): 2388. doi: 10.3390/su16062388 DOI: https://doi.org/10.3390/su16062388
2. Davis SJ, Lewis NS, Shaner M, et al. Net-zero emissions energy systems. Science, 2018, 360(6396): eaas9793. doi: 10.1126/science.aas9793 DOI: https://doi.org/10.1126/science.aas9793
3. Dunn S. Hydrogen futures: toward a sustainable energy system. International journal of hydrogen energy, 2002, 27(3): 235-264. doi: 10.1016/S0360-3199(01)00131-8 DOI: https://doi.org/10.1016/S0360-3199(01)00131-8
4. Evro S, Oni BA, Tomomewo OS. Carbon neutrality and hydrogen energy systems. International Journal of Hydrogen Energy, 2024, 78: 1449-1467. doi: 10.1016/j.ijhydene.2024.06.407 DOI: https://doi.org/10.1016/j.ijhydene.2024.06.407
5. Midilli A, Ay M, Dincer I, et al. On hydrogen and hydrogen energy strategies: I: current status and needs. Renewable and sustainable energy reviews, 2005, 9(3): 255-271. doi: 10.1016/j.rser.2004.05.003 DOI: https://doi.org/10.1016/j.rser.2004.05.003
6. Peschka W. Liquid hydrogen: fuel of the future, 1st ed. Springer Vienna; 2012.
7. Liu Z, Hao J, Yu Z, et al. Multi-condition sensitivity analysis of proton exchange membrane electrolysis cell using a segmented diagnostic method. Clean Energy Science and Technology, 2025, 3(3): 459. doi: 10.18686/cest459 DOI: https://doi.org/10.18686/cest459
8. Abe JO, Popoola API, Ajenifuja E, et al. Hydrogen energy, economy and storage: Review and recommendation. International journal of hydrogen energy, 2019, 44(29): 15072-15086. doi: 10.1016/j.ijhydene.2019.04.068 DOI: https://doi.org/10.1016/j.ijhydene.2019.04.068
9. Barthélémy H, Weber M, Barbier F. Hydrogen storage: Recent improvements and industrial perspectives. International journal of hydrogen energy, 2017, 42(11): 7254-7262. doi: 10.1016/j.ijhydene.2016.03.178 DOI: https://doi.org/10.1016/j.ijhydene.2016.03.178
10. Bi Y, Ju Y. Review on cryogenic technologies for CO2 removal from natural gas. Frontiers in Energy, 2022, 16(5): 793-811. doi: 10.1007/s11708-022-0821-0 DOI: https://doi.org/10.1007/s11708-022-0821-0
11. Leachman JW, Jacobsen RT, Penoncello SG, et al. Fundamental equations of state for parahydrogen, normal hydrogen, and orthohydrogen. Journal of Physical and Chemical Reference Data, 2009, 38(3): 721-748. doi: 10.1063/1.3160306 DOI: https://doi.org/10.1063/1.3160306
12. Ma H, Sun Z, Xue Z, et al. A systemic review of hydrogen supply chain in energy transition. Frontiers in Energy, 2023, 17(1): 102-122. doi: 10.1007/s11708-023-0861-0 DOI: https://doi.org/10.1007/s11708-023-0861-0
13. Sapnken FE, Posso F, Tamba JG. Hydrogen fuel and the Belgian transport sector: a critical assessment from an environmental and sustainable development perspective. International Journal of Hydrogen Energy, 2023, 48(73): 28247-28261. doi: 10.1016/j.ijhydene.2023.04.059 DOI: https://doi.org/10.1016/j.ijhydene.2023.04.059
14. Zhang F, Zhao P, Niu M, et al. The survey of key technologies in hydrogen energy storage. International journal of hydrogen energy, 2016, 41(33): 14535-14552. doi: 10.1016/j.ijhydene.2016.05.293 DOI: https://doi.org/10.1016/j.ijhydene.2016.05.293
15. Aasadnia M, Mehrpooya M. Large-scale liquid hydrogen production methods and approaches: A review. Applied energy, 2018, 212: 57-83. doi: 10.1016/j.apenergy.2017.12.033 DOI: https://doi.org/10.1016/j.apenergy.2017.12.033
16. Al Ghafri SZS, Munro S, Cardella U, et al. Hydrogen liquefaction: a review of the fundamental physics, engineering practice and future opportunities. Energy & environmental science, 2022, 15(7): 2690-2731. doi: 10.1039/D2EE00099G DOI: https://doi.org/10.1039/D2EE00099G
17. Zhuo H, Zhao Z, Shen Z, et al. Research progress on the catalytic conversion of ortho- to para-hydrogen. CIESC Journal, 2024, 75(11): 3883-3895. doi: 10.11949/0438-1157.20241097
18. Harkness RW, Deming WE. The equilibrium of para and ortho hydrogen. Journal of the American Chemical Society, 1932, 54(7): 2850-2852. doi: 10.1021/ja01346a503 DOI: https://doi.org/10.1021/ja01346a503
19. Ilisca E, Legrand AP. Theoretical rates and correlation functions in ortho-para H2 conversion on paramagnetic surfaces. Physical Review B, 1972, 5(12): 4994. doi: 10.1103/PhysRevB.5.4994 DOI: https://doi.org/10.1103/PhysRevB.5.4994
20. Larsen AH, Simon FE, Swenson C A. The rate of evaporation of liquid hydrogen due to the ortho-para hydrogen conversion. Review of Scientific Instruments, 1948, 19(4): 266-269. doi: 10.1063/1.1741241 DOI: https://doi.org/10.1063/1.1741241
21. Leachman J, Street MJ, Graham T. Catalytic pressurization of liquid hydrogen fuel tanks for unmanned aerial vehicles. In: AIP Conference Proceedings. American Institute of Physics; 2012. Volume 1434 No. 1, pp. 1261-1267. doi: 10.1063/1.4707049 DOI: https://doi.org/10.1063/1.4707049
22. Woolley HW, Scott RB, Brickwedde FG. Compilation of thermal properties of hydrogen in its various isotopic and ortho-para modifications. Journal of Research of the National Bureau of Standards, 1948, 41: 379-475. DOI: https://doi.org/10.6028/jres.041.037
23. Ionex® Type OP Catalyst Technical Datasheet. Available online: https://www.molecularproducts.com/wp-content/uploads/2023/08/179_Rev_F-SDS-Ionex-Type-O-P-Catalyst.pdf (accessed on 9 October 2025).
24. Ionex Type OP catalyst. Available online: https://www.molecularproducts.com/products/ionex-type-op-catalyst (accessed on 9 October 2025).
25. Abe H, Mizoguchi H, Eguchi R, et al. Exploration of heterogeneous catalyst for molecular hydrogen ortho‐para conversion[C]//Exploration. 2024, 4(3): 20230040. doi: 10.1002/EXP.20230040 DOI: https://doi.org/10.1002/EXP.20230040
26. Pan W, Xing S, Xiao J, et al. Geometric configuration dependent ortho-to-para hydrogen conversion over pure α-Fe2O3 catalysts. CrystEngComm, 2025. doi: 10.1039/D5CE00460H DOI: https://doi.org/10.1039/D5CE00460H
27. Xu H, Lu Y, Song L, et al. A stable-performance ortho-to para-hydrogen conversion catalyst MIL-Br@ MS. Journal of Physics: Conference Series. IOP Publishing, 2025, 3080(1): 012135. doi: 10.1088/1742-6596/3080/1/012135 DOI: https://doi.org/10.1088/1742-6596/3080/1/012135
28. Xue M, Xu H, Shen J, et al. A high specific surface area and amorphous cobalt oxide@ molecular sieve supported catalyst for ortho-to para-hydrogen conversion. International Journal of Hydrogen Energy, 2025, 103: 341-348. doi: 10.1016/j.ijhydene.2025.01.091 DOI: https://doi.org/10.1016/j.ijhydene.2025.01.091
29. Zhou H, Li Z, Wu Q, et al. A high-precision experimental measurement system and method for the parahydrogen concentration and the Ortho-Para hydrogen catalyst's catalytic performance. Gas Science and Engineering, 2024, 125: 205260. doi: 10.1016/j.jgsce.2024.205260 DOI: https://doi.org/10.1016/j.jgsce.2024.205260
30. Das T, Kweon SC, Choi JG, et al. Spin conversion of hydrogen over LaFeO3/Al2O3 catalysts at low temperature: Synthesis, characterization and activity. International Journal of Hydrogen Energy, 2015, 40(1): 383-391. doi: 10.1016/j.ijhydene.2014.10.137 DOI: https://doi.org/10.1016/j.ijhydene.2014.10.137
31. Das T, Kweon SC, Nah IW, et al. Spin conversion of hydrogen using supported iron catalysts at cryogenic temperature. Cryogenics, 2015, 69: 36-43. doi: 10.1016/j.cryogenics.2015.03.003 DOI: https://doi.org/10.1016/j.cryogenics.2015.03.003
32. Das T, Nah IW, Choi JG, et al. Synthesis of iron oxide catalysts using various methods for the spin conversion of hydrogen. Reaction Kinetics, Mechanisms and Catalysis, 2016, 118(2): 669-681. doi: 10.1007/s11144-016-1035-4 DOI: https://doi.org/10.1007/s11144-016-1035-4
33. Kim JH, Kang SW, Nah IW, et al. Synthesis and characterization of Fe-modified zeolite for spin conversion of hydrogen at cryogenic temperature. International Journal of Hydrogen Energy, 2015, 40(45): 15529-15533. doi: 10.1016/j.ijhydene.2015.09.087 DOI: https://doi.org/10.1016/j.ijhydene.2015.09.087
34. Wang J, Yue C, Zhang X, et al. Doping-induced Structural Transformations in Maghemite for Enhanced Ortho-Para Hydrogen Conversion. Catalysis Today, 2025, 452: 115243. doi: 10.1016/j.cattod.2025.115243 DOI: https://doi.org/10.1016/j.cattod.2025.115243
35. Xu H, Bi S, Xue M, et al. Amorphous cobalt iron oxide nanoparticles with high magnetization intensity for spin conversion of hydrogen at 77K. International Journal of Hydrogen Energy, 2023, 48(81): 31643-31652. doi: 10.1016/j.ijhydene.2023.04.313 DOI: https://doi.org/10.1016/j.ijhydene.2023.04.313
36. Xu H, Wang J, Han Y, et al. Effect of unpaired electron number elements (Al, Cr, Mn) doping in Fe2O3 on ortho to para hydrogen conversion at 77 K. Journal of Energy Storage, 2023, 74: 109512. doi: 10.1016/j.est.2023.109512 DOI: https://doi.org/10.1016/j.est.2023.109512
37. Xu H, Wang J, Han Y, et al. Ortho-to Para-Hydrogen Spin Conversion Performance of Ho-Fe2O3 Catalytic at 77 K. In: World Hydrogen Technology Convention. Singapore; 2023. pp. 186-194. doi: 10.1007/978-981-99-8581-4_20 DOI: https://doi.org/10.1007/978-981-99-8581-4_20
38. Li G, Han R, Xu X, et al. Facile synthesis of Mn-doped hollow Fe2O3 nanospheres coated with polypyrrole as anodes for high-performance lithium-ion batteries. Rsc Advances, 2016, 6(53): 48199-48204. doi: 10.1039/C6RA08740J DOI: https://doi.org/10.1039/C6RA08740J
39. Lin Z, He M, Liu Y, et al. Effect of calcination temperature on the structural and formaldehyde removal activity of Mn/Fe2O3 catalysts. Research on Chemical Intermediates, 2021, 47(8): 3245-3261. doi: 10.1007/s11164-021-04470-2
40. Qiu L, Wu Z, Liu Y, et al. Mn Doping at High‐Activity Octahedral Vacancies of γ‐Fe2O3 for Oxygen Reduction Reaction Electrocatalysis in Metal‐Air Batteries. Angewandte Chemie, 2025, 137(12): e202421918. doi: 10.1002/ange.202421918 DOI: https://doi.org/10.1002/ange.202421918
41. Yin S, Zhu B, Sun Y, et al. Effect of Mn addition on the low‐temperature NH3‐selective catalytic reduction of NOx over Fe2O3/activated coke catalysts: Experiment and mechanism. Asia‐Pacific Journal of Chemical Engineering, 2018, 13(5): e2231. doi: 10.1002/apj.2231 DOI: https://doi.org/10.1002/apj.2231
42. Yuan Q, Li P, Liu J, et al. Facet-dependent selective adsorption of Mn-doped α-Fe2O3 nanocrystals toward heavy-metal ions. Chemistry of Materials, 2017, 29(23): 10198-10205. doi: 10.1021/acs.chemmater.7b04114 DOI: https://doi.org/10.1021/acs.chemmater.7b04114
43. Yu X, Wang J, Shen J, et al. Impact of effective magnetic moment of manganese oxides with different oxidation states on the ortho-para hydrogen conversion at 77 K. Journal of Catalysis, 2025: 116412. doi: 10.1016/j.jcat.2025.116412 DOI: https://doi.org/10.1016/j.jcat.2025.116412
44. Chen Y, Zhuo H, Shen Z, et al. Catalytic Mechanism Studies of Ortho-para H2 Conversion Over Iron Oxide Catalysts. EcoEnergy, 2025: e70004. doi: 10.1002/ece2.70004 DOI: https://doi.org/10.1002/ece2.70004
45. Zhao Z, Li A, Chen Y, et al. Insights into the facet dependent conversion of ortho-to para-hydrogen over α-Fe2O3 nanocrystals. International Journal of Hydrogen Energy, 2025, 123: 281-289. doi: 10.1016/j.ijhydene.2025.03.359 DOI: https://doi.org/10.1016/j.ijhydene.2025.03.359
46. Chen L, Yang X, Chen J, et al. Continuous shape-and spectroscopy-tuning of hematite nanocrystals. Inorganic chemistry, 2010, 49(18): 8411-8420. doi: 10.1021/ic100919a DOI: https://doi.org/10.1021/ic100919a
47. Liu R, Jiang Y, Fan H, et al. Metal Ions Induce Growth and Magnetism Alternation of α‐Fe2O3 Crystals Bound by High‐Index Facets. Chemistry–A European Journal, 2012, 18(29): 8957-8963. doi: 10.1002/chem.201201108 DOI: https://doi.org/10.1002/chem.201201108
48. Mitra S, Das S, Mandal K, et al. Synthesis of a α-Fe2O3 nanocrystal in its different morphological attributes: growth mechanism, optical and magnetic properties. Nanotechnology, 2007, 18(27): 275608. doi: 10.1088/0957-4484/18/27/275608 DOI: https://doi.org/10.1088/0957-4484/18/27/275608
49. Ren X, Wang H, Wang L, et al. Water-induced stacking of α-Fe2O3 hexagonal nanoplates along the [001] direction and their facet-dependent catalytic performances. CrystEngComm, 2022, 24(37): 6512-6518. doi: 10.1039/D2CE00945E DOI: https://doi.org/10.1039/D2CE00945E
50. Zhao P, Wu F, Kronawitter CX, et al. The (0001) surfaces of α-Fe2O3 nanocrystals are preferentially activated for water oxidation by Ni doping. Physical Chemistry Chemical Physics, 2015, 17(40): 26797-26803. doi: 10.1039/C5CP04555J DOI: https://doi.org/10.1039/C5CP04555J
51. Zhou X, Lan J, Liu G, et al. Facet‐mediated photodegradation of organic dye over hematite architectures by visible light. Angewandte Chemie International Edition, 2012, 51(1): 178-182. doi: 10.1002/anie.201105028 DOI: https://doi.org/10.1002/anie.201105028
52. Zong M, Zhang X, Wang Y, et al. Synthesis of 2D hexagonal hematite nanosheets and the crystal growth mechanism. Inorganic Chemistry, 2019, 58(24): 16727-16735. doi: 10.1021/acs.inorgchem.9b02883 DOI: https://doi.org/10.1021/acs.inorgchem.9b02883
53. Gates-Rector S, Blanton T. The powder diffraction file: a quality materials characterization database. Powder diffraction, 2019, 34(4): 352-360. doi: 10.1017/S0885715619000812 DOI: https://doi.org/10.1017/S0885715619000812
54. Bhattacharya S, Dinda D, Kumar EM, et al. Charge transfer induced ferromagnetism and anomalous temperature increment of coercivity in ultrathin α-Fe2O3 decorated graphene 2D nanostructures. Journal of Applied Physics, 2019, 125(23). doi: 10.1063/1.5096396 DOI: https://doi.org/10.1063/1.5096396
55. Biswal S, Bhaskaram DS, Govindaraj G. Role of graphene oxide in modifying magnetism in α-Fe2O3 nanoparticles: Raman and magnetization studies. Materials Chemistry and Physics, 2021, 266: 124531. doi: 10.1016/j.matchemphys.2021.124531 DOI: https://doi.org/10.1016/j.matchemphys.2021.124531
56. Marshall CP, Dufresne WJB, Rufledt CJ. Polarized Raman spectra of hematite and assignment of external modes. Journal of Raman Spectroscopy, 2020, 51(9): 1522-1529. doi: 10.1002/jrs.5824 DOI: https://doi.org/10.1002/jrs.5824
57. Modesto Lopez LB, Pasteris JD, Biswas P. Sensitivity of micro-Raman spectrum to crystallite size of electrospray-deposited and post-annealed films of iron-oxide nanoparticle suspensions. Applied spectroscopy, 2009, 63(6): 627-635. doi: 10.1366/000370209788559539 DOI: https://doi.org/10.1366/000370209788559539
58. Marshall CP, Olcott Marshall A. Raman hyperspectral imaging of microfossils: potential pitfalls. Astrobiology, 2013, 13(10): 920-931. doi: 10.1089/ast.2013.1034 DOI: https://doi.org/10.1089/ast.2013.1034
59. Shim SH, Duffy T. Raman spectroscopy of Fe2O3 to 62 GPa, American Mineralogist, 2002, 87(2-3). doi: 10.2138/am-2002-2-314 DOI: https://doi.org/10.2138/am-2002-2-314
60. Guo X, Sun Z, Ge H, et al. MnOx bound on oxidized multi-walled carbon nanotubes as anode for lithium-ion batteries. Chemical Engineering Journal, 2021, 426: 131335. doi: 10.1016/j.cej.2021.131335 DOI: https://doi.org/10.1016/j.cej.2021.131335
61. Lin Z, He M, Liu Y, et al. Effect of calcination temperature on the structural and formaldehyde removal activity of Mn/Fe2O3 catalysts. Research on Chemical Intermediates, 2021, 47(8): 3245-3261. doi: 10.1007/s11164-021-04470-2 DOI: https://doi.org/10.1007/s11164-021-04470-2
62. Sun P, Guo R, Liu S, et al. The enhanced performance of MnOx catalyst for NH3-SCR reaction by the modification with Eu. Applied Catalysis A: General, 2017, 531: 129-138. doi: 10.1016/j.apcata.2016.10.027 DOI: https://doi.org/10.1016/j.apcata.2016.10.027




.jpg)
.jpg)

