Multi-condition sensitivity analysis of proton exchange membrane electrolysis cell using a segmented diagnostic method
DOI:
https://doi.org/10.18686/cest459Keywords:
proton exchange membrane electrolysis cell; segmented diagnostic method; current density distribution; multi-conditions; sensitivity analysisAbstract
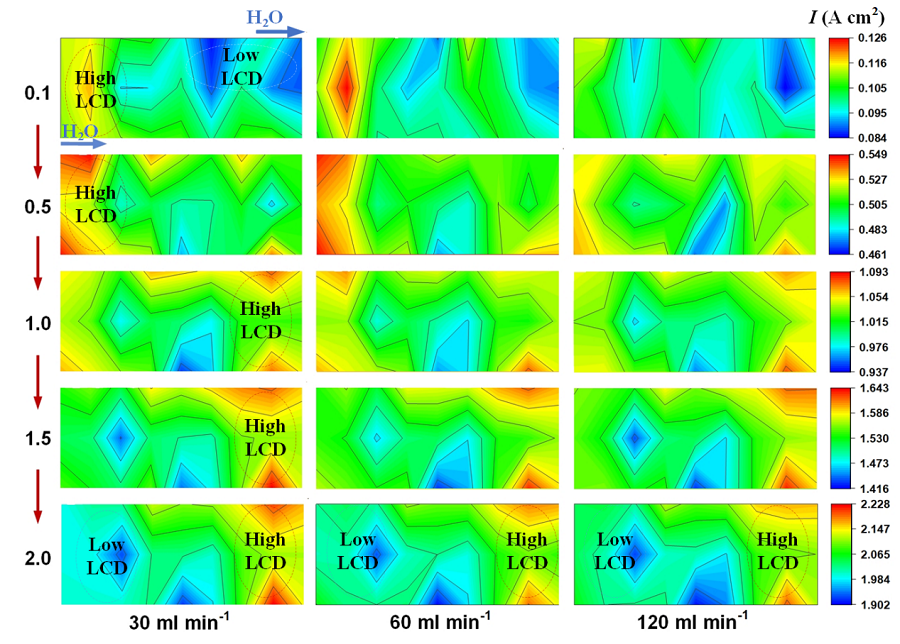
Understanding the distributions of electrochemical reaction, current density and temperature is important to improve the performance of proton exchange membrane electrolysis cell (PEMEC). Therefore, this study developed a PEMEC testing platform based on a segmented diagnostic technique and systematically analyzed the performance sensitivity and uniformity distribution of the electrolysis cell under varying water flow rates, operating temperatures, and bolt torques. The results indicate that the operating temperature exhibits the highest performance sensitivity while the water flow rate has lowest sensitivity to the PEMEC performance. Increasing water flow rate improves the bubble removal and uniformity distributions of current density and temperature, resulting in improved performance at high current density. A high bolt torque reduces contact resistance and increases uniformity distributions inside electrolyzer, thereby reducing the ohmic losses and output voltage. The elevated temperatures enhance electrochemical kinetics, heat production and uniformity distributions of current density and temperature, indicating performance improvement. The current study consolidates the understanding of influencing mechanisms of different operating conditions on distribution characteristics of multiple physical fields, contributing to enhance electrolyzer performance.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Author(s)

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
1. Atyabi SA, Afshari E. Three-dimensional multiphase model of proton exchange membrane fuel cell with honeycomb flow field at the cathode side. Journal of Cleaner Production. 2019; 214: 738-748. doi: 10.1016/j.jclepro.2018.12.293 DOI: https://doi.org/10.1016/j.jclepro.2018.12.293
2. Jiao K, Xuan J, Du Q, et al. Designing the next generation of proton-exchange membrane fuel cells. Nature. 2021; 595(7867): 361-369. doi: 10.1038/s41586-021-03482-7 DOI: https://doi.org/10.1038/s41586-021-03482-7
3. Jiao K, Wang B, Du Q, et al. Water and thermal management of proton exchange membrane fuel cells. Elsevier; 2021. doi: 10.1016/C2020-0-04110-1 DOI: https://doi.org/10.1016/B978-0-323-91116-0.00006-7
4. Zhang S, Chen W. Assessing the energy transition in China towards carbon neutrality with a probabilistic framework. Nature Communications. 2022; 13(1): 87. doi: 10.1038/s41467-021-27671-0 DOI: https://doi.org/10.1038/s41467-021-27671-0
5. Jang D, Kim J, Kim D, et al. Techno-economic analysis and Monte Carlo simulation of green hydrogen production technology through various water electrolysis technologies. Energy Conversion and Management. 2022; 258: 115499. doi: 10.1016/j.enconman.2022.115499 DOI: https://doi.org/10.1016/j.enconman.2022.115499
6. Wang Y, Pang Y, Xu H, et al. PEM fuel cell and electrolysis cell technologies and hydrogen infrastructure development—A review. Energy & Environmental Science. 2022; 15(6): 2288-2328. doi: 10.1039/D2EE00790H DOI: https://doi.org/10.1039/D2EE00790H
7. Cullen DA, Neyerlin KC, Ahluwalia RK, et al. New roads and challenges for fuel cells in heavy-duty transportation. Nature Energy. 2021; 6(5): 462-474. doi: 10.1038/s41560-021-00775-z DOI: https://doi.org/10.1038/s41560-021-00775-z
8. Wang Y, Yuan H, Martinez A, et al. Polymer electrolyte membrane fuel cell and hydrogen station networks for automobiles: Status, technology, and perspectives. Advances in Applied Energy. 2021; 2: 100011. doi: 10.1016/j.adapen.2021.100011 DOI: https://doi.org/10.1016/j.adapen.2021.100011
9. Lu Z, Yuan X, Jia X, et al. High-performance proton exchange membrane employing water-insoluble hybrid formed by chemically bonding phosphotungstic acid with polydopamine. Clean Energy Science and Technology. 2024; 2: 138. doi: 10.18686/cest.v2i2.138 DOI: https://doi.org/10.18686/cest.v2i2.138
10. Wang Z, Liu Z, Fan L, et al. Application progress of small-scale proton exchange membrane fuel cell. Energy Reviews. 2023; 2(2): 100017. doi: 10.1016/j.enrev.2023.100017 DOI: https://doi.org/10.1016/j.enrev.2023.100017
11. Liu Y, Diankai Q, Xu Z, et al. Comprehensive analysis of the gradient porous transport layer for the proton-exchange membrane electrolyzer. ACS Applied Materials & Interfaces. 2024; 16(36): 47357-47367. doi: 10.1021/acsami.4c00006 DOI: https://doi.org/10.1021/acsami.4c00006
12. Henkensmeier D, Najibah M, Harms C, et al. Overview: State-of-the art commercial membranes for anion exchange membrane water electrolysis. Journal of Electrochemical Energy Conversion and Storage. 2021; 18(2): 024001-1–024001-18. doi: 10.1115/1.4047963 DOI: https://doi.org/10.1115/1.4047963
13. Ji M. Advances in layered double hydroxides for direct seawater electrolysis: Challenges, strategies, and future perspectives. Clean Energy Science and Technology. 2025; 3: 337. doi: 10.18686/cest337 DOI: https://doi.org/10.18686/cest337
14. Frensch SH, Olesen AC, Araya SS, Kær SK. Model-supported characterization of a PEM water electrolysis cell for the effect of compression. Electrochimica Acta. 2018; 263: 228-236. doi: 10.1016/j.electacta.2018.01.040 DOI: https://doi.org/10.1016/j.electacta.2018.01.040
15. Borgardt E, Giesenberg L, Reska M, et al. Impact of clamping pressure and stress relaxation on the performance of different polymer electrolyte membrane water electrolysis cell designs. International Journal of Hydrogen Energy. 2019; 44(42): 23556-23567. doi: 10.1016/j.ijhydene.2019.07.075 DOI: https://doi.org/10.1016/j.ijhydene.2019.07.075
16. Soriano RM, Rojas N, Nieto E, et al. Influence of the gasket materials on the clamping pressure distribution in a PEM water electrolyzer: Bolt torques and operation mode in pre-conditioning. International Journal of Hydrogen Energy. 2021; 46(51): 25944-25953. doi: 10.1016/j.ijhydene.2021.03.076 DOI: https://doi.org/10.1016/j.ijhydene.2021.03.076
17. Bazarah A, Majlan EH, Husaini T, et al. Factors influencing the performance and durability of polymer electrolyte membrane water electrolyzer: A review. International Journal of Hydrogen Energy. 2022; 47(85): 35976-35989. doi: 10.1016/j.ijhydene.2022.08.180 DOI: https://doi.org/10.1016/j.ijhydene.2022.08.180
18. Hu B, He S, Su X, et al. Experimental study of the effect of fastening bolts on PEMEC performance. International Journal of Hydrogen Energy. 2023; 48(90): 35050-35063. doi: 10.1016/j.ijhydene.2023.05.116 DOI: https://doi.org/10.1016/j.ijhydene.2023.05.116
19. Hu B, He S, Zhu D, et al. Study of optimization and prediction methods for PEMEC performance considering the effects of multiple operating parameters. International Journal of Hydrogen Energy. 2024; 55: 1273-1285. doi: 10.1016/j.ijhydene.2023.11.177 DOI: https://doi.org/10.1016/j.ijhydene.2023.11.177
20. Caparros Mancera JJ, Segura Manzano F, Andújar JM, et al. An optimized balance of plant for a medium-size PEM electrolyzer: design, control and physical implementation. Electronics. 2020; 9(5): 871. doi: 10.3390/electronics9050871 DOI: https://doi.org/10.3390/electronics9050871
21. Zhang Z, Xing X. Simulation and experiment of heat and mass transfer in a proton exchange membrane electrolysis cell. International Journal of Hydrogen Energy. 2020; 45(39): 20184-20193. doi: 10.1016/j.ijhydene.2020.02.102 DOI: https://doi.org/10.1016/j.ijhydene.2020.02.102
22. Kaya MF, Demir N, Rees NV, El-Kharouf A. Improving PEM water electrolyser’s performance by magnetic field application. Applied Energy. 2020; 264: 114721. doi: 10.1016/j.apenergy.2020.114721 DOI: https://doi.org/10.1016/j.apenergy.2020.114721
23. Choi Y, Lee W, Na Y. Effect of gravity and various operating conditions on proton exchange membrane water electrolysis cell performance. Membranes. 2021; 11(11): 822. doi: 10.3390/membranes11110822 DOI: https://doi.org/10.3390/membranes11110822
24. Roenning FH, Roy A, Aaron DS, Mench MW. Mass transport limitations in polymer electrolyte water electrolyzers using spatially-resolved current measurement. Journal of Power Sources. 2022; 542: 231749. doi: 10.1016/j.jpowsour.2022.231749 DOI: https://doi.org/10.1016/j.jpowsour.2022.231749
25. Noor Azam AMI, Li NK, Zulkefli NN, et al. Parametric study and electrocatalyst of polymer electrolyte membrane (PEM) electrolysis performance. Polymers. 2023; 15(3): 560. doi: 10.3390/polym15030560 DOI: https://doi.org/10.3390/polym15030560
26. Möckl M, Bernt M, Schröter J, Jossen A. Proton exchange membrane water electrolysis at high current densities: Investigation of thermal limitations. International Journal of Hydrogen Energy. 2020; 45(3): 1417-1428. doi: 10.1016/j.ijhydene.2019.11.144 DOI: https://doi.org/10.1016/j.ijhydene.2019.11.144
27. Lickert T, Kiermaier ML, Bromberger K, et al. On the influence of the anodic porous transport layer on PEM electrolysis performance at high current densities. International Journal of Hydrogen Energy. 2020; 45(11): 6047-6058. doi: 10.1016/j.ijhydene.2019.12.204 DOI: https://doi.org/10.1016/j.ijhydene.2019.12.204
28. Lee C, Lee JK, George MG, et al. Reconciling temperature-dependent factors affecting mass transport losses in polymer electrolyte membrane electrolyzers. Energy Conversion and Management. 2020; 213: 112797. doi: 10.1016/j.enconman.2020.112797 DOI: https://doi.org/10.1016/j.enconman.2020.112797
29. Lee CH, Lee JK, Zhao B, et al. Temperature-dependent gas accumulation in polymer electrolyte membrane electrolyzer porous transport layers. Journal of Power Sources. 2020; 446: 227312. doi: 10.1016/j.jpowsour.2019.227312 DOI: https://doi.org/10.1016/j.jpowsour.2019.227312
30. Dong Y, Gao L, Ma S, et al. Combine impact of power quality and operating temperature on energy efficiency of proton exchange membrane electrolytic cells. IEEE Transactions on Applied Superconductivity. 2024; 34(8): 1–5. doi: 10.1109/TASC.2024.3469851 DOI: https://doi.org/10.1109/TASC.2024.3469851
31. Hu S, Guo B, Ding S, et al. A comprehensive review of alkaline water electrolysis mathematical modeling. Applied Energy. 2022; 327: 120099. doi: 10.1016/j.apenergy.2022.120099 DOI: https://doi.org/10.1016/j.apenergy.2022.120099
32. Wang Z, Wang X, Chen Z, et al. Energy and exergy analysis of a proton exchange membrane water electrolysis system without additional internal cooling. Renewable Energy. 2021; 180: 1333-1343. doi: 10.1016/j.renene.2021.09.037 DOI: https://doi.org/10.1016/j.renene.2021.09.037
33. Wang K, Feng Y, Xiao F, et al. Operando analysis of through-plane interlayer temperatures in the PEM electrolyzer cell under various operating conditions. Applied Energy. 2023; 348: 121588. doi: 10.1016/j.apenergy.2023.121588 DOI: https://doi.org/10.1016/j.apenergy.2023.121588
34. Selamet OF, Ergoktas MS. Effects of bolt torque and contact resistance on the performance of the polymer electrolyte membrane electrolyzers. Journal of Power Sources. 2015; 281: 103-113. doi: 10.1016/j.jpowsour.2015.01.162 DOI: https://doi.org/10.1016/j.jpowsour.2015.01.162




.jpg)
.jpg)

