Advances and challenges in discharge plasma-assisted catalyst synthesis and surface engineering
DOI:
https://doi.org/10.18686/cest426Keywords:
discharge plasma; catalyst preparation; catalyst modification; vapor deposition; atomic dopingAbstract
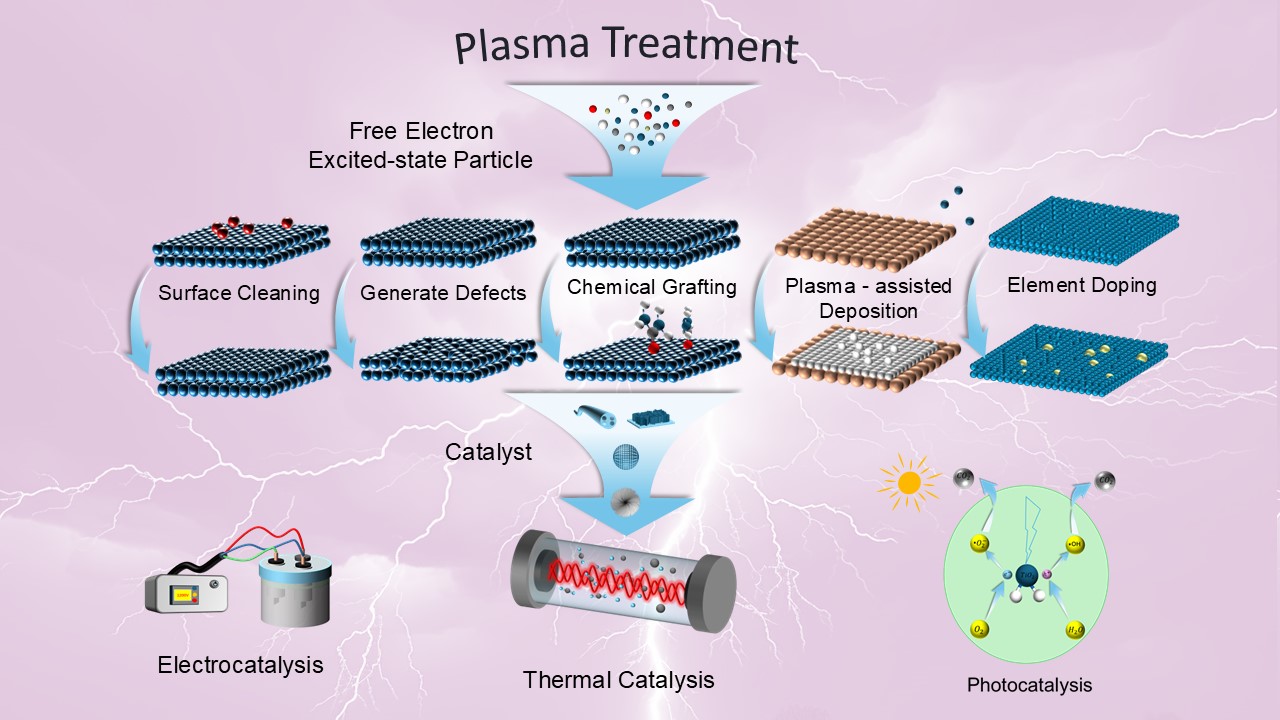
The application of discharge plasma in catalyst preparation and modification is reviewed in this paper. Catalysts play a crucial role in various fields, and discharge plasma, with its unique physicochemical properties and environmental friendliness, shows great potential in the preparation and surface engineering of catalysts. Plasma can effectively activate reactant molecules under mild conditions, thereby enhancing the reaction rate, and regulate the microstructure and active site distribution of the catalysts, thereby improving the performance of specific catalytic reactions. In this paper, different plasma sources and discharge fundamentals are reviewed, mainly emphasising on the application of plasma in catalysts preparation and surface modification. The advantages and applications of plasma-assisted catalyst synthesis, plasma chemical vapor deposition and plasma atomic layer deposition are discussed. The modification effects of plasma on the physical and chemical properties of catalysts are analyzed, and the effects of these modifications on different reaction types and their mechanisms are outlined. Finally, future research directions and challenges are discussed to offer reference for the development of discharge plasma technology in material and catalysis sciences.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Author(s)

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
1. Wang Z, Zhang Y, Neyts EC, et al. Catalyst Preparation with Plasmas: How Does It Work? ACS Catal. 2018; 8(3): 2093-2110. doi: 10.1021/acscatal.7b03723 DOI: https://doi.org/10.1021/acscatal.7b03723
2. Wang L, Wu J, Wang S, et al. The reformation of catalyst: From a trial-and-error synthesis to rational design. Nano Research. 2024; 17: 3261-3301. doi: 10.1007/s12274-023-6037-8 DOI: https://doi.org/10.1007/s12274-023-6037-8
3. Ye Z, Zhao L, Nikiforov A, et al. A review of the advances in catalyst modification using nonthermal plasma: Process, Mechanism and Applications. Advances in Colloid and Interface Science. 2022; 308: 102755. doi: 10.1016/j.cis.2022.102755 DOI: https://doi.org/10.1016/j.cis.2022.102755
4. Sun J, Qu Z, Gao Y, et al. Plasma power-to-X (PP2X): status and opportunities for non-thermal plasma technologies. Journal of Physics D: Applied Physics. 2024; 57: 503002. doi: 10.1088/1361-6463/ad7bc4 DOI: https://doi.org/10.1088/1361-6463/ad7bc4
5. Di L, Zhang J, Zhang X, et al. Cold plasma treatment of catalytic materials: a review. Journal of Physics D: Applied Physics. 2021; 54(33): 333001. doi: 10.1088/1361-6463/ac0269 DOI: https://doi.org/10.1088/1361-6463/ac0269
6. Bo Z, Yang Y, Chen J, et al. Plasma-enhanced chemical vapor deposition synthesis of vertically oriented graphene nanosheets. Nanoscale. 2013; 5(12): 5180. doi: 10.1039/c3nr33449j DOI: https://doi.org/10.1039/c3nr33449j
7. Chawdhury P, Bhargavi KVSS, Selvaraj M, et al. Promising catalytic activity by non-thermal plasma synthesized SBA-15-supported metal catalysts in one-step plasma-catalytic methane conversion to value-added fuels. Catalysis Science & Technology. 2020; 10(16): 5566-5578. doi: 10.1039/d0cy00900h DOI: https://doi.org/10.1039/D0CY00900H
8. Yang J, Zhu X, Yu Q, et al. Plasma-induced defect engineering: Boosted the reverse water gas shift reaction performance with electron trap. Journal of Colloid and Interface Science. 2020; 580: 814-821. doi: 10.1016/j.jcis.2020.07.032 DOI: https://doi.org/10.1016/j.jcis.2020.07.032
9. Sun X, Bao J, Li K, et al. Advance in Using Plasma Technology for Modification or Fabrication of Carbon‐Based Materials and Their Applications in Environmental, Material, and Energy Fields. Advanced Functional Materials. 2021; 31(7). doi: 10.1002/adfm.202006287 DOI: https://doi.org/10.1002/adfm.202006287
10. Xu J, Xia P, Zhang Q, et al. Coke resistance of Ni-based catalysts enhanced by cold plasma treatment for CH4–CO2 reforming: Review. International Journal of Hydrogen Energy. 2021; 46(45): 23174-23189. doi: 10.1016/j.ijhydene.2021.03.245 DOI: https://doi.org/10.1016/j.ijhydene.2021.03.245
11. Behrens M. Coprecipitation: An excellent tool for the synthesis of supported metal catalysts – From the understanding of the well known recipes to new materials. Catalysis Today. 2015; 246: 46-54. doi: 10.1016/j.cattod.2014.07.050 DOI: https://doi.org/10.1016/j.cattod.2014.07.050
12. Khan DT, Ho TNO, Tran TA, et al. Structural and catalytic properties of sol-gel derived iron-doped calcium cobalt oxide Ca3CO2-XFexO6. Journal of Porous Materials. 2025; 32: 717-725. DOI: https://doi.org/10.1007/s10934-024-01729-y
13. Mehrabadi BAT, Eskandari S, Khan U, et al. A Review of Preparation Methods for Supported Metal Catalysts. Advances in Catalysis. 2017; 61: 1-35. doi: 10.1016/bs.acat.2017.10.001 DOI: https://doi.org/10.1016/bs.acat.2017.10.001
14. Dou S, Tao L, Wang R, et al. Plasma‐Assisted Synthesis and Surface Modification of Electrode Materials for Renewable Energy. Advanced Materials. 2018; 30(21). doi: 10.1002/adma.201705850 DOI: https://doi.org/10.1002/adma.201705850
15. Trinh QH, Mok YS. Environmental plasma-catalysis for the energy-efficient treatment of volatile organic compounds. Korean Journal of Chemical Engineering. 2016; 33(3): 735-748. doi: 10.1007/s11814-015-0300-y DOI: https://doi.org/10.1007/s11814-015-0300-y
16. Bootluck W, Khongnakorn W, Jutaporn P, et al. Enhancement of S-scheme heterojunction hematite and titanium dioxide nanocomposite by plasma treatment in the photocatalytic membrane for hydrogen separation. Journal of Environmental Chemical Engineering. 2023; 11(5): 110674. doi: 10.1016/j.jece.2023.110674 DOI: https://doi.org/10.1016/j.jece.2023.110674
17. Huang YS, Liu YT, Perng TP, et al. Enhancing photocatalytic properties of continuous few-layer MoS2 thin films for hydrogen production by water splitting through defect engineering with Ar plasma treatment. Nano Energy. 2023; 109: 108295. doi: 10.1016/j.nanoen.2023.108295 DOI: https://doi.org/10.1016/j.nanoen.2023.108295
18. Liang X, Liu P, Qiu Z, et al. Plasma Technology for Advanced Electrochemical Energy Storage. Chemistry – A European Journal. 2024; 30(19). doi: 10.1002/chem.202304168 DOI: https://doi.org/10.1002/chem.202304168
19. Maslova V, Nastase R, Veryasov G, et al. Current status and challenges of plasma and plasma-catalysis for methane coupling: A review. Progress in Energy and Combustion Science. 2024; 101: 101096. doi: 10.1016/j.pecs.2023.101096 DOI: https://doi.org/10.1016/j.pecs.2023.101096
20. George A, Shen B, Craven M, et al. A Review of Non-Thermal Plasma Technology: A novel solution for CO2 conversion and utilization. Renewable and Sustainable Energy Reviews. 2021; 135: 109702. doi: 10.1016/j.rser.2020.109702 DOI: https://doi.org/10.1016/j.rser.2020.109702
21. Bruggeman PJ, Iza F, Brandenburg R. Foundations of atmospheric pressure non-equilibrium plasmas. Plasma Sources Science and Technology. 2017; 26(12): 123002. doi: 10.1088/1361-6595/aa97af DOI: https://doi.org/10.1088/1361-6595/aa97af
22. Li J, Liu K, Yan S, et al. Application of thermal plasma technology for the treatment of solid wastes in China: An overview. Waste Management. 2016; 58: 260-269. doi: 10.1016/j.wasman.2016.06.011 DOI: https://doi.org/10.1016/j.wasman.2016.06.011
23. Samal S. Thermal plasma technology: The prospective future in material processing. Journal of Cleaner Production. 2017; 142: 3131-3150. doi: 10.1016/j.jclepro.2016.10.154 DOI: https://doi.org/10.1016/j.jclepro.2016.10.154
24. Chen H, Mu Y, Xu S, et al. Recent advances in non-thermal plasma (NTP) catalysis towards C1 chemistry. Chinese Journal of Chemical Engineering. 2020; 28(8): 2010-2021. doi: 10.1016/j.cjche.2020.05.027 DOI: https://doi.org/10.1016/j.cjche.2020.05.027
25. Kortshagen UR, Sankaran RM, Pereira RN, et al. Nonthermal Plasma Synthesis of Nanocrystals: Fundamental Principles, Materials, and Applications. Chemical Reviews. 2016; 116(18): 11061-11127. doi: 10.1021/acs.chemrev.6b00039 DOI: https://doi.org/10.1021/acs.chemrev.6b00039
26. Ruiz-Martín M, Oliva-Ramírez M, González-Elipe AR, et al. Plasma catalysis for gas conversion – Impact of catalyst on the plasma behavior. Current Opinion in Green and Sustainable Chemistry. 2025; 51: 100990. doi: 10.1016/j.cogsc.2024.100990 DOI: https://doi.org/10.1016/j.cogsc.2024.100990
27. Ahmed N, Luo W, Zhao R, et al. Role of Plasma in Catalyst Preparation and Modification for Oxygen Evolution Reaction. Precision Chemistry. 2024; 3(3): 110-127. doi: 10.1021/prechem.4c00075 DOI: https://doi.org/10.1021/prechem.4c00075
28. Qin C, Tian S, Jiang ZJ, et al. Low temperature plasma-assisted synthesis and modification of water splitting electrocatalysts. Electrochimica Acta. 2023; 449: 142179. doi: 10.1016/j.electacta.2023.142179 DOI: https://doi.org/10.1016/j.electacta.2023.142179
29. Snoeckx R, Bogaerts A. Plasma technology – a novel solution for CO2 conversion? Chemical Society Reviews. 2017; 46(19): 5805-5863. doi: 10.1039/c6cs00066e DOI: https://doi.org/10.1039/C6CS00066E
30. He J, Wen X, Wu L, et al. Dielectric barrier discharge plasma for nanomaterials: Fabrication, modification and analytical applications. TrAC Trends in Analytical Chemistry. 2022; 156: 116715. doi: 10.1016/j.trac.2022.116715 DOI: https://doi.org/10.1016/j.trac.2022.116715
31. Zhang Q, Wang J, Guo F, et al. Nitrogen cold plasma treatment stabilizes Cu0/Cu+ electrocatalysts to enhance CO2 to C2 conversion. Journal of Energy Chemistry. 2023; 84: 321-328. doi: 10.1016/j.jechem.2023.05.008 DOI: https://doi.org/10.1016/j.jechem.2023.05.008
32. Ozkan A, Dufour T, Silva T, et al. DBD in burst mode: solution for more efficient CO2 conversion? Plasma Sources Science and Technology. 2016; 25(5): 055005. doi: 10.1088/0963-0252/25/5/055005 DOI: https://doi.org/10.1088/0963-0252/25/5/055005
33. Fridman A, Nester S, Kennedy LA, et al. Gliding arc gas discharge. Progress in Energy and Combustion Science.1999; 25(2): 211-231. doi: 10.1016/S0360-1285(98)00021-5 DOI: https://doi.org/10.1016/S0360-1285(98)00021-5
34. Mutaf‐Yardimci O, Saveliev AV, Porshnev PI, et al. Non‐Equilibrium Effects in Gliding Arc Dischargesa. Annals of the New York Academy of Sciences. 1999; 891(1): 304-308. doi: 10.1111/j.1749-6632.1999.tb08777.x DOI: https://doi.org/10.1111/j.1749-6632.1999.tb08777.x
35. Shin DH, Bang CU, Kim JH, et al. Modification of metal surfaces by microwave plasma at atmospheric pressure. Surface and Coatings Technology. 2007; 201(9-11): 4939-4942. doi: 10.1016/j.surfcoat.2006.07.073 DOI: https://doi.org/10.1016/j.surfcoat.2006.07.073
36. Ashford B, Tu X. Non-thermal plasma technology for the conversion of CO2. Current Opinion in Green and Sustainable Chemistry. 2017; 3: 45-49. doi: 10.1016/j.cogsc.2016.12.001 DOI: https://doi.org/10.1016/j.cogsc.2016.12.001
37. Keshri AK, Agarwal A. Plasma Processing of Nanomaterials for Functional Applications—A Review. Nanoscience and Nanotechnology Letters. 2012; 4(3): 228-250. doi: 10.1166/nnl.2012.1324 DOI: https://doi.org/10.1166/nnl.2012.1324
38. Yu F, Liu M, Ma C, et al. A Review on the Promising Plasma-Assisted Preparation of Electrocatalysts. Nanomaterials. 2019; 9(10): 1436. doi: 10.3390/nano9101436 DOI: https://doi.org/10.3390/nano9101436
39. Anastas PT. Meeting the challenges to sustainability through green chemistry. Green Chemistry. 2003; 5(2): G29–G34. doi: 10.1039/b211620k DOI: https://doi.org/10.1039/b211620k
40. Taghvaei H, Heravi M, Rahimpour MR. Synthesis of supported nanocatalysts via novel non‐thermal plasma methods and its application in catalytic processes. Plasma Processes and Polymers. 2017; 14(6). doi: 10.1002/ppap.201600204 DOI: https://doi.org/10.1002/ppap.201600204
41. Rao P, Yu Y, Wang S, et al. Understanding the improvement mechanism of plasma etching treatment on oxygen reduction reaction catalysts. Exploration. 2024; 4(1). DOI: https://doi.org/10.1002/EXP.20230034
42. Oh JH, Lee YH, Kim M, et al. Evaluation of thermal plasma-synthesized cobalt boride nanoparticles as efficient water-splitting catalysts. Journal of Environmental Chemical Engineering. 2023; 11(2): 109578. doi: 10.1016/j.jece.2023.109578 DOI: https://doi.org/10.1016/j.jece.2023.109578
43. Shaolong L, Yuchen L, Qiang C, et al. Preparation of Pt/CNT catalyst with high dispersion structure via plasma jet. Diamond and Related Materials. 2024; 141: 110674. doi: 10.1016/j.diamond.2023.110674 DOI: https://doi.org/10.1016/j.diamond.2023.110674
44. Lüsi M, Erikson H, Treshchalov A, et al. Oxygen reduction reaction on Pd nanocatalysts prepared by plasma-assisted synthesis on different carbon nanomaterials. Nanotechnology. 2020; 32(3): 035401. doi: 10.1088/1361-6528/abbd6f DOI: https://doi.org/10.1088/1361-6528/abbd6f
45. Yi K, Liu D, Chen X, et al. Plasma-Enhanced Chemical Vapor Deposition of Two-Dimensional Materials for Applications. Accounts of Chemical Research. 2021; 54(4): 1011-1022. doi: 10.1021/acs.accounts.0c00757 DOI: https://doi.org/10.1021/acs.accounts.0c00757
46. de Freitas ASM, Maciel CC, Rodrigues JS, et al. Organosilicon films deposited in low-pressure plasma from hexamethyldisiloxane — A review. Vacuum. 2021; 194: 110556. doi: 10.1016/j.vacuum.2021.110556 DOI: https://doi.org/10.1016/j.vacuum.2021.110556
47. Kierzkowska-Pawlak H, Kruszczak E, Tyczkowski J. Catalytic activity of plasma-deposited Co3O4-based thin films for CO2 hydration – A new approach to carbon capture applications. Applied Catalysis B: Environmental. 2022; 304: 120961. doi: 10.1016/j.apcatb.2021.120961 DOI: https://doi.org/10.1016/j.apcatb.2021.120961
48. Song S, Guo M, Zhang S, et al. Plasma-assisted synthesis of hierarchical NiCoxPy nanosheets as robust and stable electrocatalyst for hydrogen evolution reaction in both acidic and alkaline media. Electrochimica Acta. 2020; 331: 135431. doi: 10.1016/j.electacta.2019.135431 DOI: https://doi.org/10.1016/j.electacta.2019.135431
49. Bora J, Basumatary B, Podder S, et al. A substrate constituent Na-catalyzed growth of carbon nanotubes on glass substrate by atmospheric pressure PECVD. Applied Surface Science. 2024; 648: 158988. doi: 10.1016/j.apsusc.2023.158988 DOI: https://doi.org/10.1016/j.apsusc.2023.158988
50. Bigiani L, Gasparotto A, Andreu T, et al. Au–Manganese Oxide Nanostructures by a Plasma‐Assisted Process as Electrocatalysts for Oxygen Evolution: A Chemico‐Physical Investigation. Advanced Sustainable Systems. 2020; 5(11). doi: 10.1002/adsu.202000177 DOI: https://doi.org/10.1002/adsu.202000177
51. Paramanik B, Das D. Tailoring the morphology of vertically aligned carbon nanorod arrays grown on Co catalyst nanoparticles and using MW-PECVD. Ceramics International. 2024; 50(18): 33915-33925. doi: 10.1016/j.ceramint.2024.06.211 DOI: https://doi.org/10.1016/j.ceramint.2024.06.211
52. Wu S, Huang D, Yu H, et al. Molecular understanding of the effect of hydrogen on graphene growth by plasma-enhanced chemical vapor deposition. Physical Chemistry Chemical Physics. 2022; 24(17): 10297-10304. doi: 10.1039/d1cp04510e DOI: https://doi.org/10.1039/D1CP04510E
53. Fronczak M, Similska M, Ziółkowski B, et al. Novel non-metallic carbon-nitrogen photocatalysts deposited in cold plasma for hydrogen production. International Journal of Hydrogen Energy. 2024; 81: 263-269. doi: 10.1016/j.ijhydene.2024.07.299 DOI: https://doi.org/10.1016/j.ijhydene.2024.07.299
54. Zhu X, Shen Y, Florea I, et al. Synthesis of Sn-catalyzed Ge nanowires and Ge/Si heterostructures via a gradient method. Materials Letters. 2025; 379: 137674. doi: 10.1016/j.matlet.2024.137674 DOI: https://doi.org/10.1016/j.matlet.2024.137674
55. Shi H, Wu W, Wei F, et al. Three elements for the preparation of vertical graphene by RF-PECVD method. FlatChem. 2021; 30: 100306. doi: 10.1016/j.flatc.2021.100306 DOI: https://doi.org/10.1016/j.flatc.2021.100306
56. Lenef JD, Lee SY, Fuelling KM, et al. Atomic Layer Deposition of Cu Electrocatalysts on Gas Diffusion Electrodes for CO2 Reduction. Nano Letters. 2023; 23(23): 10779-10787. doi: 10.1021/acs.nanolett.3c02917 DOI: https://doi.org/10.1021/acs.nanolett.3c02917
57. Tang S, Tian D, Li Z, et al. Preparation of palladium-based catalyst by plasma-assisted atomic layer deposition and its applications in CO2 hydrogenation reduction. Plasma Science and Technology. 2024; 26(6): 064004. doi: 10.1088/2058-6272/ad1fd9 DOI: https://doi.org/10.1088/2058-6272/ad1fd9
58. Tian X, Wang D, Ouyang B, et al. Plasma-assisted fluidized-bed atomic layer deposition of Pd-Cu nanoparticles on porous powder for CO2 hydrogenation. Plasma Sources Science and Technology. 2023; 32(4): 045010. doi: 10.1088/1361-6595/acc54c DOI: https://doi.org/10.1088/1361-6595/acc54c
59. Haghverdi Khamene S, van Helvoirt C, Tsampas MN, et al. Electrochemical Activation of Atomic-Layer-Deposited Nickel Oxide for Water Oxidation. The Journal of Physical Chemistry C. 2023; 127(46): 22570-22582. doi: 10.1021/acs.jpcc.3c05002 DOI: https://doi.org/10.1021/acs.jpcc.3c05002
60. Lemago H, Addin F, Kárajz D, et al. Synthesis of TiO2/Al2O3 Double-Layer Inverse Opal by Thermal and Plasma-Assisted Atomic Layer Deposition for Photocatalytic Applications. Nanomaterials. 2023; 13(8): 1314. doi: 10.3390/nano13081314 DOI: https://doi.org/10.3390/nano13081314
61. Geerts L, Ramachandran RK, Dendooven J, et al. Creation of gallium acid and platinum metal sites in bifunctional zeolite hydroisomerization and hydrocracking catalysts by atomic layer deposition. Catalysis Science & Technology. 2020; 10(6): 1778-1788. doi: 10.1039/c9cy02610j DOI: https://doi.org/10.1039/C9CY02610J
62. Blomme R, Ramesh R, Henderick L, et al. Atomic layer deposition for tuning the surface chemical composition of nickel iron phosphates for oxygen evolution reaction in alkaline electrolyzers. Nanotechnology. 2024; 35(23): 235401. doi: 10.1088/1361-6528/ad2e48 DOI: https://doi.org/10.1088/1361-6528/ad2e48
63. Kim DJ, Jeong HJ, Shim JW, et al. Improving the Stability of Polymer Electrolyte Membrane Fuel Cells via Atomic Layer-Deposited Cerium Oxide. International Journal of Energy Research. 2023; 2023: 1-9. doi: 10.1155/2023/5506063 DOI: https://doi.org/10.1155/2023/5506063
64. Lee JH, Lund IN, Eisenbraun ET, et al. Silicide-induced multi-wall carbon nanotube growth on silicon nanowires. Nanotechnology. 2011; 22(8): 085603. doi: 10.1088/0957-4484/22/8/085603 DOI: https://doi.org/10.1088/0957-4484/22/8/085603
65. Lee YS, Shim JW, Choi YS, et al. Stabilization of platinum catalyst surface‐treated by atomic layer deposition of cobalt for polymer electrolyte membrane fuel cells. International Journal of Energy Research; 2022. DOI: https://doi.org/10.1002/er.8239
66. Ramesh R, Han S, Nandi DK, et al. Ultralow Loading (Single‐Atom and Clusters) of the Pt Catalyst by Atomic Layer Deposition Using Dimethyl ((3,4‐η) N,N‐dimethyl‐3‐butene‐1‐amine‐N) Platinum (DDAP) on the High‐Surface‐Area Substrate for Hydrogen Evolution Reaction. Advanced Materials Interfaces. 2021; 8(3). doi: 10.1002/admi.202001508 DOI: https://doi.org/10.1002/admi.202001508
67. Kye S, Kim HJ, Go D, et al. Ultralow-Loading Ruthenium Catalysts by Plasma-Enhanced Atomic Layer Deposition for a Solid Oxide Fuel Cell. ACS Catalysis. 2021; 11(6): 3523–3529. doi: 10.1021/acscatal.0c04526 DOI: https://doi.org/10.1021/acscatal.0c04526
68. Li J, Ma C, Zhu S, et al. A Review of Recent Advances of Dielectric Barrier Discharge Plasma in Catalysis. Nanomaterials. 2019; 9(10): 1428. doi: 10.3390/nano9101428 DOI: https://doi.org/10.3390/nano9101428
69. Gholami P, Heidari A, Khataee A, et al. Oxygen and nitrogen plasma modifications of ZnCuCo LDH-graphene nanocomposites for photocatalytic hydrogen production and antibiotic degradation. Sep. Purif. Technol. 2023; 325; 124706. DOI: https://doi.org/10.1016/j.seppur.2023.124706
70. Luo Y,Wu Y, Huang C, et al. Plasma modified and tailored defective electrocatalysts for water electrolysis and hydrogen fuel cells. EcoMat. 2022; 4: e12197. DOI: https://doi.org/10.1002/eom2.12197
71. Chen S, Wang H, Dong F. Activation and characterization of environmental catalysts in plasma-catalysis: Status and challenges. Journal of Hazardous Materials. 2022; 427: 128150. doi: 10.1016/j.jhazmat.2021.128150 DOI: https://doi.org/10.1016/j.jhazmat.2021.128150
72. Pinard L, Batiot-Dupeyrat C. Non-thermal plasma for catalyst regeneration: A review. Catalysis Today. 2024; 426: 114372. doi: 10.1016/j.cattod.2023.114372 DOI: https://doi.org/10.1016/j.cattod.2023.114372
73. Jin Y, Ren C, Yang L, Wang D. Comparative Study of the Surface Cleaning for Ar-/He-Based Plasma Jets at Atmospheric Pressure. IEEE Transactions on Plasma Science. 2015; 43(9): 1-1. doi: 10.1109/TPS.2015.2459080 DOI: https://doi.org/10.1109/TPS.2015.2459080
74. Park J, Cho I, Jeon H, et al. Conversion of Layered WS2 Crystals into Mixed‐Domain Electrochemical Catalysts by Plasma‐Assisted Surface Reconstruction. Advanced Materials. 2024; 36(25). doi: 10.1002/adma.202314031 DOI: https://doi.org/10.1002/adma.202314031
75. Taylor GN, Wolf TM. Oxygen plasma removal of thin polymer films. Polymer Engineering & Science. 1980; 20(16): 1087-1092. doi: 10.1002/pen.760201610 DOI: https://doi.org/10.1002/pen.760201610
76. Rodríguez-Villanueva C, Encinas N, Abenojar J, et al. Assessment of atmospheric plasma treatment cleaning effect on steel surfaces. Surface and Coatings Technology. 2013; 236: 450-456. doi: 10.1016/j.surfcoat.2013.10.036 DOI: https://doi.org/10.1016/j.surfcoat.2013.10.036
77. Zhang S, Han W, Hu X, et al. Supported bimetallic hydrogenation catalysts treated by non-thermal plasmas. Catalysis Today. 2023; 418: 114076. doi: 10.1016/j.cattod.2023.114076 DOI: https://doi.org/10.1016/j.cattod.2023.114076
78. Giannakaris N, Niebauer M, Gürtler G, et al. Surface cleaning with atmospheric pressure plasma jet investigated by in-situ optical emission spectroscopy and laser-induced breakdown spectroscopy. Applied Surface Science. 2025; 684: 161751. doi: 10.1016/j.apsusc.2024.161751 DOI: https://doi.org/10.1016/j.apsusc.2024.161751
79. Babu R, Das MK. Effects of surface-active agents on bubble growth and detachment from submerged orifice. Chemical Engineering Science. 2018; 179: 172-184. doi: 10.1016/j.ces.2018.01.028 DOI: https://doi.org/10.1016/j.ces.2018.01.028
80. Chen Q, Song B, Li X, et al. Enhancing the Properties of Photocatalysts via Nonthermal Plasma Modification: Recent Advances, Treatment Variables, Mechanisms, and Perspectives. Industrial & Engineering Chemistry Research. 2021; 60(47): 16813-16826. doi: 10.1021/acs.iecr.1c03062 DOI: https://doi.org/10.1021/acs.iecr.1c03062
81. Kim SG, Kim SY, Lee HW. Effect of ammonia gas etching on growth of vertically aligned carbon nanotubes/nanofibers. Transactions of Nonferrous Metals Society of China. 2011; 21(Supplement 1): s130-s134. doi: 10.1016/S1003-6326(11)61076-5 DOI: https://doi.org/10.1016/S1003-6326(11)61076-5
82. Liao, Q. et al. Plasma-Induced Surface Reconstruction of NiFe/Co3O4 Nanoarrays for High-Current and Ultrastable Oxygen Evolution and the Urea Oxidation Reaction. Ind. Eng. Chem. Res. 61, 16050–16060 (2022) DOI: https://doi.org/10.1021/acs.iecr.2c02958
83. Rao P, Wu D, Qin YY, et al. Facile fabrication of single-atom catalysts by a plasma-etching strategy for oxygen reduction reaction. Journal of Materials Chemistry A. 2022; 10(12): 6531-6537. doi: 10.1039/d1ta09154a DOI: https://doi.org/10.1039/D1TA09154A
84. Sun JB, Peimyoo N, Douglas JO, et al. Doping density, not valency, influences catalytic metal-assisted plasma etching of silicon. Materials Horizons. 2023; 10(9): 3393-3403. doi: 10.1039/d3mh00649b DOI: https://doi.org/10.1039/D3MH00649B
85. Raizada P, Soni V, Kumar A, et al. Surface defect engineering of metal oxides photocatalyst for energy application and water treatment. Journal of Materiomics. 2021; 7(2): 388-418. doi: 10.1016/j.jmat.2020.10.009 DOI: https://doi.org/10.1016/j.jmat.2020.10.009
86. Zheng J, Wang Z. Defect engineering for surface reconstruction of metal oxide catalysts during OER. Chem Catalysis. 2024; 4(11): 101091. doi: 10.1016/j.checat.2024.101091 DOI: https://doi.org/10.1016/j.checat.2024.101091
87. Zhong W, Chen J, Zhang P, et al. Air plasma etching towards rich active sites in Fe/N-porous carbon for the oxygen reduction reaction with superior catalytic performance. Journal of Materials Chemistry A. 2017; 5(32): 16605-16610. doi: 10.1039/c7ta05035f DOI: https://doi.org/10.1039/C7TA05035F
88. Liu S, Yin S, Zhang Z, et al. Regulation of defects and nitrogen species on carbon nanotube by plasma-etching for peroxymonosulfate activation: Inducing non-radical/radical oxidation of organic contaminants. Journal of Hazardous Materials. 2023; 441: 129905. doi: 10.1016/j.jhazmat.2022.129905 DOI: https://doi.org/10.1016/j.jhazmat.2022.129905
89. Rahemi N, Haghighi M, Babaluo AA, et al. Synthesis and physicochemical characterizations of Ni/Al2O3–ZrO2 nanocatalyst prepared via impregnation method and treated with non-thermal plasma for CO2 reforming of CH4. Journal of Industrial and Engineering Chemistry. 2013; 19(5): 1566-1576. doi: 10.1016/j.jiec.2013.01.024 DOI: https://doi.org/10.1016/j.jiec.2013.01.024
90. Li K, Tang X, Yi H, et al. Research on manganese oxide catalysts surface pretreated with non-thermal plasma for NO catalytic oxidation capacity enhancement. Applied Surface Science. 2013; 264: 557-562. doi: 10.1016/j.apsusc.2012.10.064 DOI: https://doi.org/10.1016/j.apsusc.2012.10.064
91. Petrović M, Jovanović T, Rančev S, et al. Plasma modified electrosynthesized cerium oxide catalyst for plasma and photocatalytic degradation of RB 19 dye. Journal of Environmental Chemical Engineering. 2022; 10(3): 107931. doi: 10.1016/j.jece.2022.107931 DOI: https://doi.org/10.1016/j.jece.2022.107931
92. Shi L, Zhou Y, Tan X, et al. Dielectric barrier discharge plasma grafting carboxylate groups on Pt/Al2O3 catalysts for highly efficient hydrogen release from perhydro-dibenzyltoluene. Catalysis Science & Technology. 2022; 12(5): 1441-1449. doi: 10.1039/d1cy01652k DOI: https://doi.org/10.1039/D1CY01652K
93. Lu N, Jiang X, Zhu Y, et al. Single-Atom-Layer Metallization of Plasmonic Semiconductor Surface for Selectively Enhancing IR-Driven Photocatalytic Reduction of CO2 into CH4. Advanced Materials. 2024; 37(4). doi: 10.1002/adma.202413931 DOI: https://doi.org/10.1002/adma.202413931
94. Magureanu M, Mandache NB, Gherendi F, et al. Improvement of catalytic activity of graphene oxide by plasma treatment. Catalysis Today. 2021; 366: 2-9. doi: 10.1016/j.cattod.2020.07.022 DOI: https://doi.org/10.1016/j.cattod.2020.07.022
95. Lai J, Sunderland B, Xue J, et al. Study on hydrophilicity of polymer surfaces improved by plasma treatment. Applied Surface Science. 2006; 252(10): 3375-3379. doi: 10.1016/j.apsusc.2005.05.038 DOI: https://doi.org/10.1016/j.apsusc.2005.05.038
96. Lei MK, Liu Y, Li YP. Controllable wettability of poly(ethylene terephthlate) film modified by oxygen combined inductively and capacitively coupled radio-frequency plasma. Applied Surface Science. 2011; 257(16): 7350-7358. doi: 10.1016/j.apsusc.2011.03.145 DOI: https://doi.org/10.1016/j.apsusc.2011.03.145
97. Bao W, Lu K, Fu P, et al. Solution plasma-assisted synthesis of oxyhydroxides for advanced electrocatalytic water splitting. Chemical Engineering Journal. 2023; 474: 145826. doi: 10.1016/j.cej.2023.145826 DOI: https://doi.org/10.1016/j.cej.2023.145826
98. Wang Y, Zhou W, Shuai Y, et al. Tuning the Pt-N coordination in Pt/MOFs nanosheets under N2 plasma for enhanced oxygen reduction reaction. Journal of Alloys and Compounds. 2023; 968: 171915. doi: 10.1016/j.jallcom.2023.171915 DOI: https://doi.org/10.1016/j.jallcom.2023.171915
99. Zhao, K. et al. Boosting HER performance by using plasma prepared N-doped CNTs to support Pt nanoparticles. Int. J. Hydrog. Energy 90, 1271–1278 (2024) DOI: https://doi.org/10.1016/j.ijhydene.2024.10.103
100. Wang R, Sun X, Zhong J, et al. Low-temperature plasma-assisted synthesis of iron and nitrogen co-doped CoFeP-N nanowires for high-efficiency electrocatalytic water splitting. Applied Catalysis B: Environment and Energy. 2024; 352: 124027. doi: 10.1016/j.apcatb.2024.124027 DOI: https://doi.org/10.1016/j.apcatb.2024.124027
101. Zhang D, Wang F, Zhao W, et al. Boosting Hydrogen Evolution Reaction Activity of Amorphous Molybdenum Sulfide Under High Currents Via Preferential Electron Filling Induced by Tungsten Doping. Advanced Science. 2022; 9(27). doi: 10.1002/advs.202202445 DOI: https://doi.org/10.1002/advs.202202445
102. Khlyustova A, Sirotkin N, Kusova T, et al. Doped TiO2: the effect of doping elements on photocatalytic activity. Materials Advances. 2020; 1(5): 1193-1201. doi: 10.1039/d0ma00171f DOI: https://doi.org/10.1039/D0MA00171F
103. Zhang J, Nie W, Wang R, et al. Plasma technique regulates the electronic structure and dual functional catalytic performance of p-VNiCoPy/NiFeOx heterojunction catalysts for hydrogen production through overall water splitting. Journal of Alloys and Compounds. 2024; 1004: 175900. doi: 10.1016/j.jallcom.2024.175900 DOI: https://doi.org/10.1016/j.jallcom.2024.175900
104. Lian HY, Geng S, Wang YZ, et al. Plasma-enhanced methanol steam reforming over Pt/In2O3-ZnO/Al2O3 catalyst with ultralow Pt-loading for in-situ H2 production. Chemical Engineering Journal. 2025; 503: 158479. doi: 10.1016/j.cej.2024.158479 DOI: https://doi.org/10.1016/j.cej.2024.158479
105. Rahman TU, Roy H, Fariha A, et al. Progress in plasma doping semiconductor photocatalysts for efficient pollutant remediation and hydrogen generation. Separation and Purification Technology. 2023; 320: 124141. doi: 10.1016/j.seppur.2023.124141 DOI: https://doi.org/10.1016/j.seppur.2023.124141
106. Dou S, Tao L, Huo J, et al. Etched and doped Co9S8/graphene hybrid for oxygen electrocatalysis. Energy & Environmental Science. 2016; 9(4): 1320-1326. doi: 10.1039/c6ee00054a DOI: https://doi.org/10.1039/C6EE00054A
107. Son H, Kim S, Lee JH, et al. Dye-synthesized N, S co-doped carbon via plasma engineering as metal-free oxygen reduction reaction electrocatalysts. Journal of Physics D: Applied Physics. 2021; 55(7): 074001. doi: 10.1088/1361-6463/ac30ff DOI: https://doi.org/10.1088/1361-6463/ac30ff
108. Xu A, Dong C, Wu A, et al. Plasma-modified C-doped Co3O4 nanosheets for the oxygen evolution reaction designed by Butler–Volmer and first-principle calculations. Journal of Materials Chemistry A. 2019; 7(9): 4581-4595. doi: 10.1039/c8ta11424b DOI: https://doi.org/10.1039/C8TA11424B
109. Al-Naggar AH, Shinde NM, Kim JS, et al. Water splitting performance of metal and non-metal-doped transition metal oxide electrocatalysts. Coordination Chemistry Reviews. 2023; 474: 214864. doi: 10.1016/j.ccr.2022.214864 DOI: https://doi.org/10.1016/j.ccr.2022.214864
110. Li L, Xu R, Zhang X, et al. Inducing oxygen vacancies using plasma etching to enhance the oxygen evolution reaction activity of the CoMn2O4 catalyst. Ceramics International. 2024; 50(22): 45242-45250. doi: 10.1016/j.ceramint.2024.08.364 DOI: https://doi.org/10.1016/j.ceramint.2024.08.364
111. Yan Y, Lin J, Cao J, et al. Activating and optimizing the activity of NiCoP nanosheets for electrocatalytic alkaline water splitting through the V doping effect enhanced by P vacancies. Journal of Materials Chemistry A. 2019; 7(42): 24486-24492. doi: 10.1039/c9ta09283h DOI: https://doi.org/10.1039/C9TA09283H
112. Fan L, Zhang B, He T, et al. Low temperature and rapid synthesis of nitrogen-doped carbon-based catalysts via atmospheric pressure plasma. Journal of Physics D: Applied Physics. 2023; 56(49): 495202. doi: 10.1088/1361-6463/acf6ce DOI: https://doi.org/10.1088/1361-6463/acf6ce
113. Lu Y, Ma A, Yu Y, et al. Engineering Oxygen Vacancies into LaCoO3 Perovskite for Efficient Electrocatalytic Oxygen Evolution. ACS Sustainable Chemistry & Engineering. 2018; 7(3): 2906-2910. doi: 10.1021/acssuschemeng.8b05717 DOI: https://doi.org/10.1021/acssuschemeng.8b05717
114. Chen G, Xiang H, Guo Y, et al. Yttrium‐ and nitrogen‐doped NiCo phosphide nanosheets for high‐efficiency water electrolysis. Carbon Energy. 2024; 6(8). doi: 10.1002/cey2.522 DOI: https://doi.org/10.1002/cey2.522
115. Rui N, Zhang X, Zhang F, et al. Highly active Ni/CeO2 catalyst for CO2 methanation: Preparation and characterization. Applied Catalysis B: Environmental. 2021; 282: 119581. doi: 10.1016/j.apcatb.2020.119581 DOI: https://doi.org/10.1016/j.apcatb.2020.119581
116. Qian S, Chen Y, Yan B, et al. Plasma treated M1 MoVNbTeO –CeO2 composite catalyst for improved performance of oxidative dehydrogenation of ethane. Green Energy & Environment. 2023; 8(3): 904-914. doi: 10.1016/j.gee.2022.01.001 DOI: https://doi.org/10.1016/j.gee.2022.01.001
117. Wang X, Fan G, Guo S, et al. Regulated Dual Defects of Bridging Organic and Terminal Inorganic Ligands in Iron‐based Metal‐Organic Framework Nodes for Efficient Photocatalytic Ammonia Synthesis. Angewandte Chemie International Edition. 2024; 63(22). doi: 10.1002/anie.202404258 DOI: https://doi.org/10.1002/anie.202404258
118. Yan Y, Lin J, Xu T, et al. Atomic‐Level Platinum Filling into Ni‐Vacancies of Dual‐Deficient NiO for Boosting Electrocatalytic Hydrogen Evolution. Advanced Energy Materials. 2022; 12(24). doi: 10.1002/aenm.202200434 DOI: https://doi.org/10.1002/aenm.202200434
119. Zeng K, Li W, Zhou Y, et al. Multilayer hollow MnCo2O4 microsphere with oxygen vacancies as efficient electrocatalyst for oxygen evolution reaction. Chemical Engineering Journal. 2021; 421: 127831. doi: 10.1016/j.cej.2020.127831 DOI: https://doi.org/10.1016/j.cej.2020.127831
120. Chen L, Zhou W, Huo C, et al. Improved metal-support interaction in Ru/CeO2 catalyst via plasma-treated strategy for dichloroethane oxidation. Applied Catalysis A: General. 2023; 660: 119215. doi: 10.1016/j.apcata.2023.119215 DOI: https://doi.org/10.1016/j.apcata.2023.119215
121. Li G, Wu X, Guo H, et al. Plasma Transforming Ni(OH)2 Nanosheets into Porous Nickel Nitride Sheets for Alkaline Hydrogen Evolution. ACS Applied Materials & Interfaces. 2020; 12(5): 5951-5957. doi: 10.1021/acsami.9b20887 DOI: https://doi.org/10.1021/acsami.9b20887
122. Zhang T, Wu J, Chen J, et al. Activating Titanium Metal with H2 Plasma for the Hydrogen Evolution Reaction. ACS Applied Materials & Interfaces. 2021; 13(21): 24682-24691. doi: 10.1021/acsami.1c02646 DOI: https://doi.org/10.1021/acsami.1c02646
123. Liu X, Chen G, Guo Y, et al. Fabric-like rhodium-nickel-tungsten oxide nanosheets for highly-efficient electrocatalytic H2 generation in an alkaline electrolyte. Journal of Colloid and Interface Science. 2024; 659: 895-904. doi: 10.1016/j.jcis.2024.01.060 DOI: https://doi.org/10.1016/j.jcis.2024.01.060
124. Qin C, Tian S, Wu J, et al. Plasma‐Assisted Surface Engineering to Stabilize Mn3+ in Electrodeposited Manganese Oxide Films for Water Oxidation. ChemCatChem. 2024; 16(22). doi: 10.1002/cctc.202401033 DOI: https://doi.org/10.1002/cctc.202401033
125. Liu R, Chen G, Guo Y, et al. Designing persimmon-liked FeOOH-(CrCo)Ox on the plasma-treated cobalt foam for a highly efficient oxygen evolution in an alkaline-seawater electrolyte. Chemical Engineering Journal. 2024; 500: 157098. doi: 10.1016/j.cej.2024.157098 DOI: https://doi.org/10.1016/j.cej.2024.157098
126. Hu T, Chen W, Liu Y, et al. Plasma‐Induced Formation of Pt Nanoparticles with Optimized Surface Oxidation States for Methanol Oxidation and Oxygen Reduction Reactions to Achieve High‐Performance DMFCs. Small. 2023; 19(46). doi: 10.1002/smll.202304076 DOI: https://doi.org/10.1002/smll.202304076
127. Li H, Wang J, Tjardts T, et al. Plasma‐Engineering of Oxygen Vacancies on NiCO2O4 Nanowires with Enhanced Bifunctional Electrocatalytic Performance for Rechargeable Zinc‐air Battery. Small. 2024; 20(24): e2310660. doi: 10.1002/smll.202310660 DOI: https://doi.org/10.1002/smll.202310660
128. Han H, Jin S, Park S, et al. Plasma-induced oxygen vacancies in amorphous MnOx boost catalytic performance for electrochemical CO2 reduction. Nano Energy. 2021; 79: 105492. doi: 10.1016/j.nanoen.2020.105492 DOI: https://doi.org/10.1016/j.nanoen.2020.105492
129. Yu Q, Guo C, Ge J, et al. Morphology controlling of silver by plasma engineering for electrocatalytic carbon dioxide reduction. Journal of Power Sources. 2020; 453: 227846. doi: 10.1016/j.jpowsour.2020.227846 DOI: https://doi.org/10.1016/j.jpowsour.2020.227846
130. Scholten F, Sinev I, Bernal MI, Cuenya BR. Plasma-Modified Dendritic Cu Catalyst for CO2 Electroreduction. ACS Catalysis. 2019; 9(6): 5496–5502. doi: 10.1021/acscatal.9b00483 DOI: https://doi.org/10.1021/acscatal.9b00483
131. Zhao Y, Ge H, Kondo Y, et al. Photosynthesis of hydrogen peroxide in a two-phase system by hydrophobic Au nanoparticle-deposited plasmonic TiO2 catalysts. Catalysis Today. 2024; 431: 114558. doi: 10.1016/j.cattod.2024.114558 DOI: https://doi.org/10.1016/j.cattod.2024.114558
132. Gan G, Li Y, Zhang G. Advances in the design of plasmonic photocatalysts for enhanced photocatalytic CO2 reduction. Separation and Purification Technology. 2025; 355: 129587. doi: 10.1016/j.seppur.2024.129587 DOI: https://doi.org/10.1016/j.seppur.2024.129587
133. Ding J, Sun X, Wang Q, et al. Plasma synthesis of Pt/g-C3N4 photocatalysts with enhanced photocatalytic hydrogen generation. Journal of Alloys and Compounds. 2021; 873: 159871. doi: 10.1016/j.jallcom.2021.159871 DOI: https://doi.org/10.1016/j.jallcom.2021.159871
134. Yuan Y, Zhou J, Bayles A, et al. Steam methane reforming using a regenerable antenna-reactor plasmonic photocatalyst. Nature Catalysis. 2024; 7: 1339–1349. DOI: https://doi.org/10.1038/s41929-024-01248-8
135. Chen B, Wang B, Sun Y, et al. Plasma-Assisted Surface Interactions of Pt/CeO2 Catalyst for Enhanced Toluene Catalytic Oxidation. Catalysts. 2018; 9(1): 2. doi: 10.3390/catal9010002 DOI: https://doi.org/10.3390/catal9010002
136. Reiser D, von Keudell A. Reaction Mechanisms and Plasma-Catalyst Interaction in Plasma-Assisted Oxidation of n-Butane: A Data-Driven Approach. Plasma Chemistry and Plasma Processing. 2024; 44(2): 867-890. doi: 10.1007/s11090-023-10443-7 DOI: https://doi.org/10.1007/s11090-023-10443-7
137. Lin TK, Wuu DS, Huang SY, et al. Preparation and Characterization of Sprayed-Yttrium Oxyfluoride Corrosion Protective Coating for Plasma Process Chambers. Coatings. 2018; 8(10): 373. doi: 10.3390/coatings8100373 DOI: https://doi.org/10.3390/coatings8100373
138. Okubo M. Recent Development of Technology in Scale-up of Plasma Reactors for Environmental and Energy Applications. Plasma Chemistry and Plasma Processing. 2021; 42: 3-33. DOI: https://doi.org/10.1007/s11090-021-10201-7
139. Zhang H, Ding H, Wang W, et al. Air plasma activation of catalytic sites within MXene nanosheets for oxygen evolution and urea oxidation catalysis. Materials Today Energy. 2024; 44: 101623. doi: 10.1016/j.mtener.2024.101623 DOI: https://doi.org/10.1016/j.mtener.2024.101623
140. Li T, Gao Y, Zhou R, et al. Outlook for improving energy efficiency, conversion rates, and selectivity of plasma-assisted CO2 conversion. Current Opinion in Green and Sustainable Chemistry. 2024; 47: 100915. doi: 10.1016/j.cogsc.2024.100915 DOI: https://doi.org/10.1016/j.cogsc.2024.100915
141. Liu C, Li M, Wang J, et al. Plasma methods for preparing green catalysts: Current status and perspective. Chinese Journal of Catalysis. 2016; 37(3): 340-348. doi: 10.1016/S1872-2067(15)61020-8 DOI: https://doi.org/10.1016/S1872-2067(15)61020-8
142. Lin TK, Wuu DS, Huang SY, et al. Preparation and Characterization of Sprayed-Yttrium Oxyfluoride Corrosion Protective Coating for Plasma Process Chambers. Coatings. 2018; 8(10): 373. doi: 10.3390/coatings8100373 DOI: https://doi.org/10.3390/coatings8100373
143. Luo Y, Yue X, Zhang H, et al. Recent advances in energy efficiency optimization methods for plasma CO2 conversion. Science of The Total Environment. 2024; 906: 167486. doi: 10.1016/j.scitotenv.2023.167486 DOI: https://doi.org/10.1016/j.scitotenv.2023.167486




.jpg)
.jpg)

