Assessment issues on electrochemical catalysis for water splitting
DOI:
https://doi.org/10.18686/cest376Keywords:
electrochemical analysis; electrochemical catalysis; water electrolysis; LSV; electrolyzerAbstract
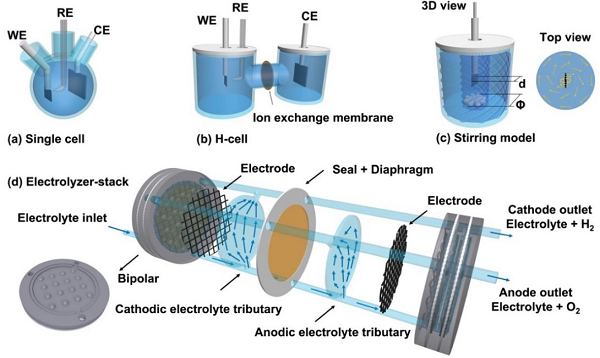
A number of catalysts have been developed for electrochemical water electrolysis in the last decades, however, the inadequate understanding and the non-standard measurements result in inaccurate activity evaluation. Especially at present, water electrolysis aims to deal with higher-current-density scenario, and the exaggeration for catalysts heavily mislead to unnecessary trial and error in the real application. The dynamic lineage sweep voltammetry, as a universal estimation method to evaluate the activity of material, is discussed, including the issues on electrochemical assessment and the possible factors to inaccuracy. Accordingly, we cast our own opinions and suggestions in practice, such as building the reasonable electrolytic cell system, including the electrode position, size, electrolyte, cell types, scan rate, stirring, selection of the counter and reference electrodes, etc. The IR compensation on resistance and conversion of specific activity are also mentioned. We suggest that the researchers should operate in a standardized manner and obtain the authentic data, without intentionally hiding the tricky experiment settings.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Author(s)

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
1. IEA. Global hydrogen review 2023. Available online: https://www.iea.org/reports/global-hydrogen-review-2023 (accessed on 5 April 2025).
2. SAMR/SAC. Testing and evaluation of electrodes performance for hydrogen production by water electrolysis. Available online: https://openstd.samr.gov.cn/bzgk/std/newGbInfo?hcno=076B7669422DBD40D83C7F5D20700CC3 (accessed on 5 April 2025).
3. SAMR/SAC. Proton exchange membrane fuel cell-Part 4: Test method for electrocalysts. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=C97A4179A2CBEE6A04471DFDCC6FE83F (accessed on 5 April 2025).
4. Bhuvanendran N, Ravichandran S, Xu Q, et al. A quick guide to the assessment of key electrochemical performance indicators for the oxygen reduction reaction: A comprehensive review. International Journal of Hydrogen Energy. 2022; 47(11): 7113-7138. doi: 10.1016/j.ijhydene.2021.12.072 DOI: https://doi.org/10.1016/j.ijhydene.2021.12.072
5. Boettcher SW, Oener SZ, Lonergan MC, et al. Potentially confusing: Potentials in electrochemistry. ACS Energy Letters. 2020; 6(1): 261-266. doi: 10.1021/acsenergylett.0c02443 DOI: https://doi.org/10.1021/acsenergylett.0c02443
6. Voiry D, Chhowalla M, Gogotsi Y, et al. Best practices for reporting electrocatalytic performance of nanomaterials. ACS Nano. 2018; 12(10): 9635-9638. doi: 10.1021/acsnano.8b07700 DOI: https://doi.org/10.1021/acsnano.8b07700
7. Zaman S, Chen S. A perspective on inaccurate measurements in oxygen reduction and carbon dioxide reduction reactions. Journal of Catalysis. 2023; 421: 221-227. doi: 10.1016/j.jcat.2023.03.030 DOI: https://doi.org/10.1016/j.jcat.2023.03.030
8. Lu X, Zhao C. Electrodeposition of hierarchically structured three-dimensional nickel-iron electrodes for efficient oxygen evolution at high current densities. Nature Communications. 2015; 6(1). doi: 10.1038/ncomms7616 DOI: https://doi.org/10.1038/ncomms7616
9. Niu S, Li S, Du Y, et al. How to reliably report the overpotential of an electrocatalyst. ACS Energy Letters. 2020; 5(4): 1083-1087. doi: 10.1021/acsenergylett.0c00321 DOI: https://doi.org/10.1021/acsenergylett.0c00321
10. Chen R, Yang C, Cai W, et al. Use of platinum as the counter electrode to study the activity of nonprecious metal catalysts for the hydrogen evolution reaction. ACS Energy Letters. 2017; 2(5): 1070-1075. doi: 10.1021/acsenergylett.7b00219 DOI: https://doi.org/10.1021/acsenergylett.7b00219
11. Jerkiewicz G. Applicability of platinum as a counter-electrode material in electrocatalysis research. ACS Catalysis. 2022; 12(4): 2661-2670. doi: 10.1021/acscatal.1c06040 DOI: https://doi.org/10.1021/acscatal.1c06040
12. Song L, Ma Y, Wang T, et al. Electrochemical “activation effect” to oxygen reduction reaction caused by long-time potential cycling process. Applied Catalysis A: General. 2019; 588: 117273. doi: 10.1016/j.apcata.2019.117273 DOI: https://doi.org/10.1016/j.apcata.2019.117273
13. Xia YF, Guo P, Li JZ, et al. How to appropriately assess the oxygen reduction reaction activity of platinum group metal catalysts with rotating disk electrode. iScience. 2021; 24(9): 103024. doi: 10.1016/j.isci.2021.103024 DOI: https://doi.org/10.1016/j.isci.2021.103024
14. Bo X, Hocking RK, Zhou S, et al. Capturing the active sites of multimetallic (oxy)hydroxides for the oxygen evolution reaction. Energy & Environmental Science. 2020; 13(11): 4225-4237. doi: 10.1039/d0ee01609h DOI: https://doi.org/10.1039/D0EE01609H
15. Bo X, Li Y, Chen X, et al. High valence chromium regulated cobalt-iron-hydroxide for enhanced water oxidation. Journal of Power Sources. 2018; 402: 381-387. doi: 10.1016/j.jpowsour.2018.09.063 DOI: https://doi.org/10.1016/j.jpowsour.2018.09.063
16. Lyu S, Guo C, Wang J, et al. Exceptional catalytic activity of oxygen evolution reaction via two-dimensional graphene multilayer confined metal-organic frameworks. Nature Communications. 2022; 13(1). doi: 10.1038/s41467-022-33847-z DOI: https://doi.org/10.1038/s41467-022-33847-z
17. Trotochaud L, Young SL, Ranney JK, et al. Nickel-Iron oxyhydroxide oxygen-evolution electrocatalysts: The role of intentional and incidental iron incorporation. Journal of the American Chemical Society. 2014; 136(18): 6744-6753. doi: 10.1021/ja502379c DOI: https://doi.org/10.1021/ja502379c
18. Zhang H, Zhang X, Zhang D, et al. One-step electrophoretic deposition of reduced graphene oxide and Ni(OH)2 composite films for controlled syntheses supercapacitor electrodes. The Journal of Physical Chemistry B. 2012; 117(6): 1616-1627. doi: 10.1021/jp305198j DOI: https://doi.org/10.1021/jp305198j
19. Liu Z, Liu Z, Zan L, et al. In situ anodic transition and cathodic contamination affect the overall voltage of alkaline water electrolysis. Molecules. 2024; 29(22): 5298. doi: 10.3390/molecules29225298 DOI: https://doi.org/10.3390/molecules29225298
20. Lee SA, Kim J, Kwon KC, et al. Anion exchange membrane water electrolysis for sustainable large‐scale hydrogen production. Carbon Neutralization. 2022; 1(1): 26-48. doi: 10.1002/cnl2.9 DOI: https://doi.org/10.1002/cnl2.9
21. Mu Y, Ma R, Xue S, et al. Recent advances and perspective on transition metal heterogeneous catalysts for efficient electrochemical water splitting. Carbon Neutralization. 2024; 3(1): 4-31. doi: 10.1002/cnl2.105 DOI: https://doi.org/10.1002/cnl2.105
22. Taibi E, Blanco H, Miranda R, et al. Green hydrogen cost reduction: scaling up electrolysers to meet the 1.50 ℃ climate goal. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2020/Dec/IRENA_Green_hydrogen_cost_2020.pdf (accessed on 5 April 2025).
23. Zhang M, Guan J, Tu Y, et al. Highly efficient conversion of surplus electricity to hydrogen energy via polysulfides redox. The Innovation. 2021; 2(3): 100144. doi: 10.1016/j.xinn.2021.100144 DOI: https://doi.org/10.1016/j.xinn.2021.100144
24. Luo Y, Zhang Z, Chhowalla M, et al. Recent advances in design of electrocatalysts for high‐current‐density water splitting. Advanced Materials. 2022; 34(16). doi: 10.1002/adma.202108133 DOI: https://doi.org/10.1002/adma.202108133
25. Bard AJ, Faulkner LR, White HS. Electrochemical methods fundamentals and applications, 3rd ed. Wiley & Sons; 2022.
26. Yu L, Ren Z. Systematic study of the influence of iR compensation on water electrolysis. Materials Today Physics. 2020; 14: 100253. doi: 10.1016/j.mtphys.2020.100253 DOI: https://doi.org/10.1016/j.mtphys.2020.100253
27. Chen S, Li W, Jiang W, et al. MOF Encapsulating N‐heterocyclic carbene‐ligated copper single‐atom site catalyst towards efficient methane electrosynthesis. Angewandte Chemie International Edition. 2021; 61(4). doi: 10.1002/anie.202114450 DOI: https://doi.org/10.1002/anie.202114450




.jpg)
.jpg)

