Impact of inlet CO2 on the performance of AEMFCs: Mechanistic insights and mitigation strategies
DOI:
https://doi.org/10.18686/cest369Keywords:
AEMFC; CO2; AEM; operational condition; pre-treatment systemAbstract
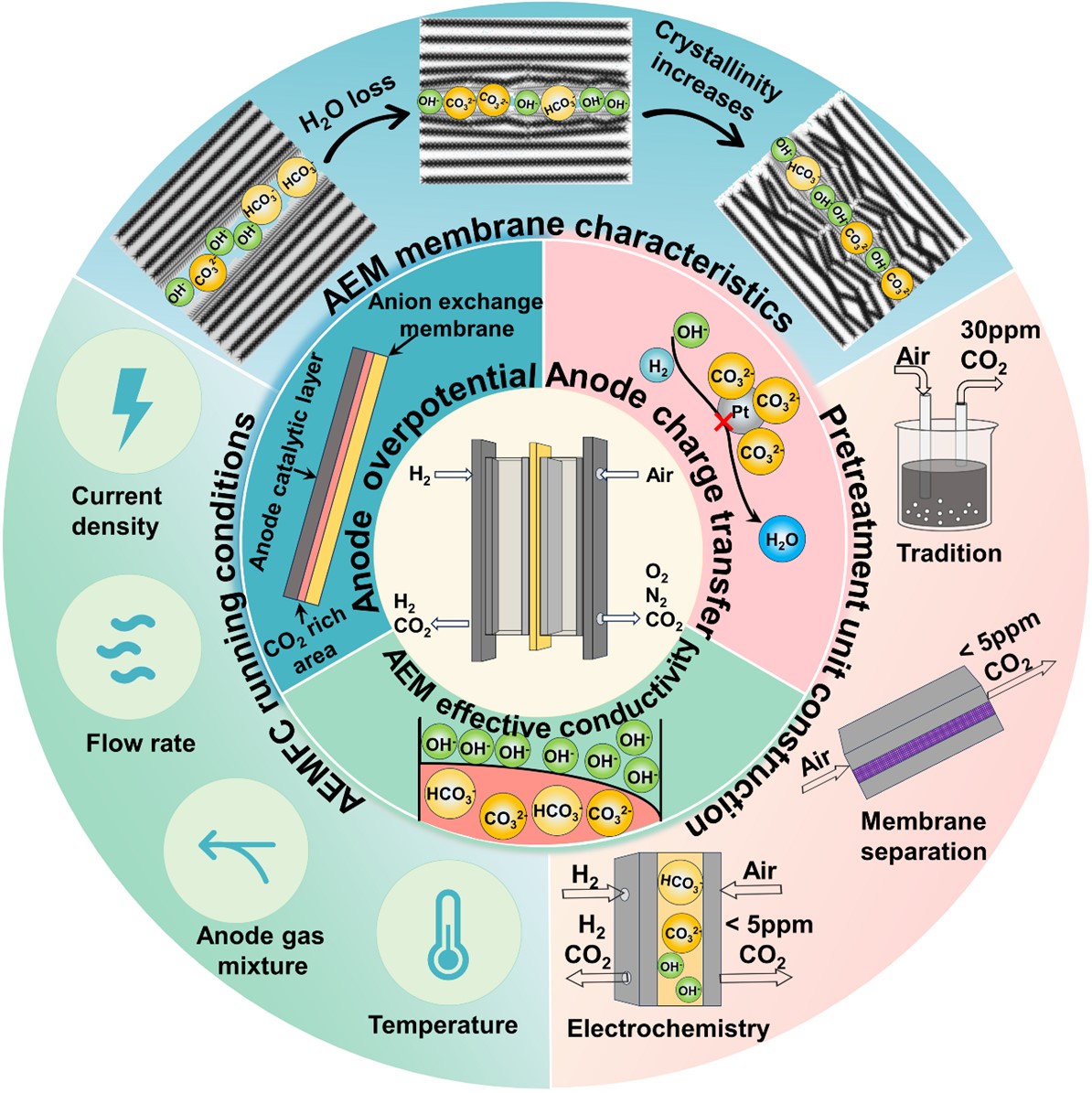
Anion exchange membrane fuel cells (AEMFCs) offer a lower assembly cost compared to proton exchange membrane fuel cells (PEMFCs), as their alkaline environment enables the use of inexpensive catalysts and bipolar plates. Currently, most performance evaluations of AEMFCs are conducted with O2 as the cathode gas. However, the ultimate goal of AEMFCs is to operate with ambient air as the cathode feed. CO2 in the air often exerts a significant negative impact on AEMFC performance, particularly by reducing the conductivity of the alkaline electrolyte and diminishing the overall efficiency of the cell. This challenge has become one of the primary barriers to the widespread adoption and optimization of AEMFC technology. This work reviews relevant studies by previous researchers and identifies three main mechanisms through which CO2 adversely affects AEMFC performance: (1) the formation of carbonate ions, which reduces the effective conductivity of the membrane; (2) the increase in anode potential, leading to voltage loss; and (3) the accumulation of carbonates, which raises the charge transfer resistance. Furthermore, this work summarizes strategies to mitigate or prevent AEMFC carbonation, focusing on membrane property modulation, operational condition optimization, and the design of the inlet air pre-treatment systems. The review aims to provide a comprehensive framework for both academic and industrial stakeholders, facilitating the advancement of AEMFC technology.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Author(s)

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
1. Mao X, Li Y, Hu X, et al. Expanded graphite (EG)/Ni@Melamine foam (MF)/EG sandwich-structured flexible bipolar plate with excellent electrical conductivity, mechanical properties, and gas permeability. Applied Energy. 2023; 338: 120929. doi: 10.1016/j.apenergy.2023.120929 DOI: https://doi.org/10.1016/j.apenergy.2023.120929
2. Guilbert D, Vitale G. Hydrogen as a Clean and Sustainable Energy Vector for Global Transition from Fossil-Based to Zero-Carbon. Clean Technologies. 2021; 3(4): 881-909. doi: 10.3390/cleantechnol3040051 DOI: https://doi.org/10.3390/cleantechnol3040051
3. Fortin P, Holdcroft S. Hydrogen Evolution at Conjugated Polymer Nanoparticle Electrodes. ECS Meeting Abstracts. 2018; MA2018-01(31): 1909-1909. doi: 10.1149/ma2018-01/31/1909 DOI: https://doi.org/10.1149/MA2018-01/31/1909
4. AlHumaidan FS, Absi Halabi M, Rana MS, et al. Blue hydrogen: Current status and future technologies. Energy Conversion and Management. 2023; 283: 116840. doi: 10.1016/j.enconman.2023.116840 DOI: https://doi.org/10.1016/j.enconman.2023.116840
5. Gencoglu MT, Ural Z. Design of a PEM fuel cell system for residential application. International Journal of Hydrogen Energy. 2009; 34(12): 5242-5248. doi: 10.1016/j.ijhydene.2008.09.038 DOI: https://doi.org/10.1016/j.ijhydene.2008.09.038
6. O’Connell M, Kolb G, Schelhaas KP, et al. Towards mass production of microstructured fuel processors for application in future distributed energy generation systems: A review of recent progress at IMM. Chemical Engineering Research and Design. 2012; 90(1): 11-18. doi: 10.1016/j.cherd.2011.08.002 DOI: https://doi.org/10.1016/j.cherd.2011.08.002
7. Mao X, Li Y, Li Y, et al. Simultaneous electrical and mechanical properties improvement for composite expanded graphite bipolar plate by incorporating surface-activated carbon black paving on the carbonized melamine foam skeleton. Composites Science and Technology. 2024; 257: 110822. doi: 10.1016/j.compscitech.2024.110822 DOI: https://doi.org/10.1016/j.compscitech.2024.110822
8. Kartha S, Grimes P. Fuel Cells: Energy Conversion for the Next Century. Physics Today. 1994; 47(11): 54-61. doi: 10.1063/1.881426 DOI: https://doi.org/10.1063/1.881426
9. Gutru R, Turtayeva Z, Xu F, et al. A comprehensive review on water management strategies and developments in anion exchange membrane fuel cells. International Journal of Hydrogen Energy. 2020; 45(38): 19642-19663. doi: 10.1016/j.ijhydene.2020.05.026 DOI: https://doi.org/10.1016/j.ijhydene.2020.05.026
10. Dekel DR, Rasin IG, Brandon S. Predicting performance stability of anion exchange membrane fuel cells. Journal of Power Sources. 2019; 420: 118-123. doi: 10.1016/j.jpowsour.2019.02.069 DOI: https://doi.org/10.1016/j.jpowsour.2019.02.069
11. Dekel DR. Review of cell performance in anion exchange membrane fuel cells. Journal of Power Sources. 2018; 375: 158-169. doi: 10.1016/j.jpowsour.2017.07.117 DOI: https://doi.org/10.1016/j.jpowsour.2017.07.117
12. Peng X, Omasta TJ, Rigdon WA, et al. Catalyst and Electrode Advances for High Performing PEM and AEM Fuel Cells. ECS Meeting Abstracts. 2017; MA2017-01(43): 1968-1968. doi: 10.1149/ma2017-01/43/1968 DOI: https://doi.org/10.1149/MA2017-01/43/1968
13. Lilloja J, Mooste M, Kibena-Põldsepp E, et al. Cobalt-, iron- and nitrogen-containing ordered mesoporous carbon-based catalysts for anion-exchange membrane fuel cell cathode. Electrochimica Acta. 2023; 439: 141676. doi: 10.1016/j.electacta.2022.141676 DOI: https://doi.org/10.1016/j.electacta.2022.141676
14. Firouzjaie HA, Mustain WE. Catalytic Advantages, Challenges, and Priorities in Alkaline Membrane Fuel Cells. ACS Catalysis. 2019; 10(1): 225-234. doi: 10.1021/acscatal.9b03892 DOI: https://doi.org/10.1021/acscatal.9b03892
15. Yassin K, Rasin IG, Willdorf-Cohen S, et al. A surprising relation between operating temperature and stability of anion exchange membrane fuel cells. Journal of Power Sources Advances. 2021; 11: 100066. doi: 10.1016/j.powera.2021.100066 DOI: https://doi.org/10.1016/j.powera.2021.100066
16. Gottesfeld S, Dekel DR, Page M, et al. Anion exchange membrane fuel cells: Current status and remaining challenges. Journal of Power Sources. 2018; 375: 170-184. doi: 10.1016/j.jpowsour.2017.08.010 DOI: https://doi.org/10.1016/j.jpowsour.2017.08.010
17. Ng W, Wong W, Rosli N, et al. Commercial Anion Exchange Membranes (AEMs) for Fuel Cell and Water Electrolyzer Applications: Performance, Durability, and Materials Advancement. Separations. 2023; 10(8): 424. doi: 10.3390/separations10080424 DOI: https://doi.org/10.3390/separations10080424
18. Omasta TJ, Wang L, Peng X, et al. Importance of balancing membrane and electrode water in anion exchange membrane fuel cells. Journal of Power Sources. 2018; 375: 205-213. doi: 10.1016/j.jpowsour.2017.05.006 DOI: https://doi.org/10.1016/j.jpowsour.2017.05.006
19. Wang L, Brink JJ, Varcoe JR. The first anion-exchange membrane fuel cell to exceed 1 W cm−2 at 70 °C with a non-Pt-group (O2) cathode. Chem Commun. 2017; 53(86): 11771-11773. doi: 10.1039/c7cc06392j DOI: https://doi.org/10.1039/C7CC06392J
20. Mustain WE, Chatenet M, Page M, et al. Durability challenges of anion exchange membrane fuel cells. Energy & Environmental Science. 2020; 13(9): 2805-2838. doi: 10.1039/d0ee01133a DOI: https://doi.org/10.1039/D0EE01133A
21. Yang W, Zhang Q, Yan Y. Membrane Electrode Assembly Preparation for Anion Exchange Membrane Fuel Cell (AEMFC): Selection of Ionomers and How to Avoid CO2 Poisoning. In: Alkaline Anion Exchange Membranes for Fuel Cells. Wiley-Vch; 2024. DOI: https://doi.org/10.1002/9783527837588.ch14
22. Kizewski J, Mudri N, Zeng R, et al. T. Slade R, Varcoe JR. Alkaline Electrolytes and Reference Electrodes for Alkaline Polymer Electrolyte Membrane Fuel Cells. ECS Transactions. 2010; 33(1): 27-35. doi: 10.1149/1.3484498 DOI: https://doi.org/10.1149/1.3484498
23. Suzuki S, Muroyama H, Matsui T, et al. Influence of CO2 dissolution into anion exchange membrane on fuel cell performance. Electrochimica Acta. 2013; 88: 552-558. DOI: https://doi.org/10.1016/j.electacta.2012.10.105
24. Ziv N, Mustain WE, Dekel DR. The Effect of Ambient Carbon Dioxide on Anion‐Exchange Membrane Fuel Cells. ChemSusChem. 2018; 11(7): 1136-1150. doi: 10.1002/cssc.201702330 DOI: https://doi.org/10.1002/cssc.201702330
25. Kiss AM, Myles TD, Grew KN, et al. Carbonate and Bicarbonate Ion Transport in Alkaline Anion Exchange Membranes. Journal of The Electrochemical Society. 2013; 160(9): F994-F999. doi: 10.1149/2.037309jes DOI: https://doi.org/10.1149/2.037309jes
26. Matsui Y, Saito M, Tasaka A, et al. Influence of Carbon Dioxide on the Performance of Anion-Exchange Membrane Fuel Cells. ECS Transactions. 2010; 25(13): 105-110. doi: 10.1149/1.3315177 DOI: https://doi.org/10.1149/1.3315177
27. Krewer U, Weinzierl C, Ziv N, et al. Impact of carbonation processes in anion exchange membrane fuel cells. Electrochimica Acta. 2018; 263: 433-446. doi: 10.1016/j.electacta.2017.12.093 DOI: https://doi.org/10.1016/j.electacta.2017.12.093
28. Balliet RJ, Newman J. Cold Start of a Polymer-Electrolyte Fuel Cell I. Development of a Two-Dimensional Model. Journal of The Electrochemical Society. 2011; 158(8): B927. doi: 10.1149/1.3592430 DOI: https://doi.org/10.1149/1.3592430
29. Zenyuk IV, Das PK, Weber AZ. Understanding Impacts of Catalyst-Layer Thickness on Fuel-Cell Performance via Mathematical Modeling. Journal of The Electrochemical Society. 2016; 163(7): F691-F703. doi: 10.1149/2.1161607jes DOI: https://doi.org/10.1149/2.1161607jes
30. Weber AZ. Effective diffusion-medium thickness for simplified polymer-electrolyte-fuel-cell modeling. Electrochimica Acta. 2008; 54(2): 311-315. doi: 10.1016/j.electacta.2008.07.084 DOI: https://doi.org/10.1016/j.electacta.2008.07.084
31. Weber AZ, Newman J. Coupled Thermal and Water Management in Polymer Electrolyte Fuel Cells. Journal of The Electrochemical Society. 2006; 153(12): A2205. doi: 10.1149/1.2352039 DOI: https://doi.org/10.1149/1.2352039
32. Gerhardt MR, Pant LM, Weber AZ. Along-the-Channel Impacts of Water Management and Carbon-Dioxide Contamination in Hydroxide-Exchange-Membrane Fuel Cells: A Modeling Study. Journal of The Electrochemical Society. 2019; 166(7): F3180-F3192. doi: 10.1149/2.0171907jes DOI: https://doi.org/10.1149/2.0171907jes
33. Inaba M, Matsui Y, Saito M, et al. Effects of Carbon Dioxide on the Performance of Anion-Exchange Membrane Fuel Cells. Electrochemistry. 2011; 79(5): 322-325. doi: 10.5796/electrochemistry.79.322 DOI: https://doi.org/10.5796/electrochemistry.79.322
34. Hyun J, Kim HT. Powering the hydrogen future: current status and challenges of anion exchange membrane fuel cells. Energy & Environmental Science. 2023; 16(12): 5633-5662. doi: 10.1039/d3ee01768k DOI: https://doi.org/10.1039/D3EE01768K
35. Li Q, Krumov MR, Hu M, et al. Origin of Performance Decline in Carbonated Anion Exchange Membrane Fuel Cells. Journal of the American Chemical Society. 2024; 146(49): 33587-33594. doi: 10.1021/jacs.4c11188 DOI: https://doi.org/10.1021/jacs.4c11188
36. Luo X, Rojas-Carbonell S, Yan Y, et al. Structure-transport relationships of poly(aryl piperidinium) anion-exchange membranes: Eeffect of anions and hydration. Journal of Membrane Science. 2020; 598: 117680. doi: 10.1016/j.memsci.2019.117680 DOI: https://doi.org/10.1016/j.memsci.2019.117680
37. Zelovich T, Simari C, Nicotera I, et al. The impact of carbonation on hydroxide diffusion in nano-confined anion exchange membranes. Journal of Materials Chemistry A. 2022; 10(20): 11137-11149. doi: 10.1039/d2ta00830k DOI: https://doi.org/10.1039/D2TA00830K
38. Divekar AG, Yang-Neyerlin AC, Antunes CM, et al. In-depth understanding of the CO2 limitation of air fed anion exchange membrane fuel cells. Sustainable Energy & Fuels. 2020; 4(4): 1801-1811. doi: 10.1039/c9se01212e DOI: https://doi.org/10.1039/C9SE01212E
39. Zheng Y, Irizarry Colón LN, Ul Hassan N, et al. Effect of Membrane Properties on the Carbonation of Anion Exchange Membrane Fuel Cells. Membranes. 2021; 11(2): 102. doi: 10.3390/membranes11020102 DOI: https://doi.org/10.3390/membranes11020102
40. Simari C, Lufrano E, Rehman MHU, et al. Effect of LDH platelets on the transport properties and carbonation of anion exchange membranes. Electrochimica Acta. 2022; 403: 139713. doi: 10.1016/j.electacta.2021.139713 DOI: https://doi.org/10.1016/j.electacta.2021.139713
41. Mandal M, Huang G, Hassan NU, et al. The Importance of Water Transport in High Conductivity and High-Power Alkaline Fuel Cells. Journal of The Electrochemical Society. 2019; 167(5): 054501. doi: 10.1149/2.0022005jes DOI: https://doi.org/10.1149/2.0022005JES
42. Adams LA, Poynton SD, Tamain C, et al. A Carbon Dioxide Tolerant Aqueous‐Electrolyte‐Free Anion‐Exchange Membrane Alkaline Fuel Cell. ChemSusChem. 2008; 1(1-2): 79-81. doi: 10.1002/cssc.200700013 DOI: https://doi.org/10.1002/cssc.200700013
43. Yanagi H, Fukuta K. Anion Exchange Membrane and Ionomer for Alkaline Membrane Fuel Cells (AMFCs). ECS Transactions. 2008; 16(2): 257-262. doi: 10.1149/1.2981860 DOI: https://doi.org/10.1149/1.2981860
44. Siroma Z, Watanabe S, Yasuda K, et al. Mathematical Modeling of the Concentration Profile of Carbonate Ions in an Anion Exchange Membrane Fuel Cell. Journal of The Electrochemical Society. 2011; 158(6): B682-B689. doi: 10.1149/1.3576120 DOI: https://doi.org/10.1149/1.3576120
45. Shiau HS, Zenyuk IV, Weber AZ. Elucidating Performance Limitations in Alkaline-Exchange- Membrane Fuel Cells. Journal of The Electrochemical Society. 2017; 164(11): E3583-E3591. doi: 10.1149/2.0531711jes DOI: https://doi.org/10.1149/2.0531711jes
46. Wrubel JA, Peracchio AA, Cassenti BN, et al. Anion Exchange Membrane Fuel Cell Performance in the Presence of Carbon Dioxide: An Investigation into the Self-Purging Mechanism. Journal of The Electrochemical Society. 2019; 166(12): F810-F820. doi: 10.1149/2.0801912jes DOI: https://doi.org/10.1149/2.0801912jes
47. Zheng Y, Huang G, Wang L, et al. Effect of reacting gas flowrates and hydration on the carbonation of anion exchange membrane fuel cells in the presence of CO2. Journal of Power Sources. 2020; 467: 228350. doi: 10.1016/j.jpowsour.2020.228350 DOI: https://doi.org/10.1016/j.jpowsour.2020.228350
48. Katayama Y, Yamauchi K, Hayashi K, et al. Anion-Exchange Membrane Fuel Cells with Improved CO2 Tolerance: Impact of Chemically Induced Bicarbonate Ion Consumption. ACS Applied Materials & Interfaces. 2017; 9(34): 28650-28658. doi: 10.1021/acsami.7b09877 DOI: https://doi.org/10.1021/acsami.7b09877
49. Grew KN, Ren X, Chu D. Effects of Temperature and Carbon Dioxide on Anion Exchange Membrane Conductivity. Electrochemical and Solid-State Letters. 2011; 14(12): B127. doi: 10.1149/2.011112esl DOI: https://doi.org/10.1149/2.011112esl
50. Zheng Y, Omasta TJ, Peng X, et al. Quantifying and elucidating the effect of CO2 on the thermodynamics, kinetics and charge transport of AEMFCs. Energy & Environmental Science. 2019; 12(9): 2806-2819. doi: 10.1039/c9ee01334b DOI: https://doi.org/10.1039/C9EE01334B
51. Merel J, Clausse M, Meunier F. Experimental Investigation on CO2 Post-Combustion Capture by Indirect Thermal Swing Adsorption Using 13X and 5A Zeolites. Industrial & Engineering Chemistry Research. 2007; 47(1): 209-215. doi: 10.1021/ie071012x DOI: https://doi.org/10.1021/ie071012x
52. Shekhah O, Belmabkhout Y, Chen Z, et al. Made-to-order metal-organic frameworks for trace carbon dioxide removal and air capture. Nature Communications. 2014; 5(1). doi: 10.1038/ncomms5228 DOI: https://doi.org/10.1038/ncomms5228
53. Shi S, Li S, Liu X. Mechanism Study of Imidazole-Type Deep Eutectic Solvents for Efficient Absorption of CO2. ACS Omega. 2022; 7(51): 48272-48281. doi: 10.1021/acsomega.2c06437 DOI: https://doi.org/10.1021/acsomega.2c06437
54. Li Y, Kong X, Zhu D, et al. Development of the DES-contained reduced graphene oxide system with efficient CO2 adsorption and photothermal desorption for pre-gas purification in AEMFCs. Separation and Purification Technology. 2025; 360: 131193. doi: 10.1016/j.seppur.2024.131193 DOI: https://doi.org/10.1016/j.seppur.2024.131193
55. Shao P, Dal-Cin MM, Guiver MD, et al. Simulation of membrane-based CO2 capture in a coal-fired power plant. Journal of Membrane Science. 2013; 427: 451-459. doi: 10.1016/j.memsci.2012.09.044 DOI: https://doi.org/10.1016/j.memsci.2012.09.044
56. Liang Z, Yang F, Li Y, et al. Designing the feasible membrane systems for CO2 removal from Air-fed Anion-Exchange membrane fuel cells. Separation and Purification Technology. 2022; 289: 120713. doi: 10.1016/j.seppur.2022.120713 DOI: https://doi.org/10.1016/j.seppur.2022.120713
57. Zhang S, Geng X, Niu C, et al. Novel mixed matrix membranes containing calixarene for enhanced CO2/N2 separation. Separation and Purification Technology. 2025; 356: 129792. doi: 10.1016/j.seppur.2024.129792 DOI: https://doi.org/10.1016/j.seppur.2024.129792
58. Wang T, Zhang Q, Lian K, et al. Fe nanoparticles confined by multiple-heteroatom-doped carbon frameworks for aqueous Zn-air battery driving CO2 electrolysis. Journal of Colloid and Interface Science. 2024; 655: 176-186. doi: 10.1016/j.jcis.2023.10.157 DOI: https://doi.org/10.1016/j.jcis.2023.10.157
59. Peng B, She H, Wei Z, et al. Sulfur-doping tunes p-d orbital coupling over asymmetric Zn-Sn dual-atom for boosting CO2 electroreduction to formate. Nature Communications. 2025; 16(1). doi: 10.1038/s41467-025-57573-4 DOI: https://doi.org/10.1038/s41467-025-57573-4
60. Zheng Y, Huang G, Mandal M, et al. Editors’ Choice—Power-Generating Electrochemical CO2 Scrubbing from Air Enabling Practical AEMFC Application. Journal of The Electrochemical Society. 2021; 168(2): 024504. doi: 10.1149/1945-7111/abe08a DOI: https://doi.org/10.1149/1945-7111/abe08a
61. Shi L, Zhao Y, Matz S, et al. A shorted membrane electrochemical cell powered by hydrogen to remove CO2 from the air feed of hydroxide exchange membrane fuel cells. Nature Energy. 2022; 7(3): 238-247. doi: 10.1038/s41560-021-00969-5 DOI: https://doi.org/10.1038/s41560-021-00969-5




.jpg)
.jpg)

