Advances in layered double hydroxides for direct seawater electrolysis: Challenges, strategies, and future perspectives
DOI:
https://doi.org/10.18686/cest337Keywords:
seawater electrolysis; oxygen evolution reaction; chloride evolution reaction; layered double hydroxides; anode catalyst; corrosion resistanceAbstract
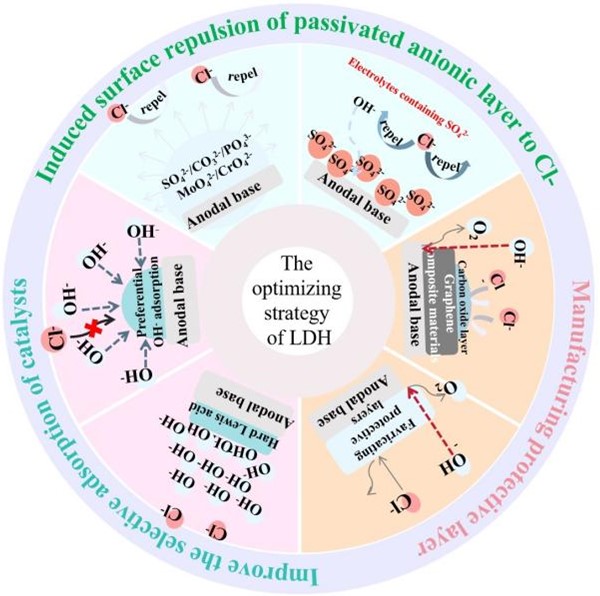
Direct electrolysis of seawater to produce hydrogen is one of the promising and low-cost ideal hydrogen production technologies. However, being different from freshwater electrolysis, seawater contains lots of ions, microorganisms, and other impurities, which make seawater electrolysis more challenging. In particular, the chloride ion in seawater usually results in a chlorine evolution reaction (CER) and competes with the oxygen evolution reaction (OER) at the anodes, the dominant rate-determining step of overall water electrolysis. In recent years, layered double hydroxides (LDHs) have attracted attention because of their excellent OER activity in alkaline solutions. In this paper, the research progress of LDHs in seawater electrolysis is reviewed, including the structure design and optimization strategies for protecting catalytic sites from Cl− corrosion, and the mechanism study to reveal the inhibition of CER during the OER process. The challenges in improving the corrosion resistance of LDHs in seawater electrolysis are concluded to provide some possible and available ways of seawater electrolysis for generating green hydrogen.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Author(s)

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
1. Chu S, Majumdar A. Opportunities and challenges for a sustainable energy future. Nature. 2012; 488(7411): 294-303. doi: 10.1038/nature11475 DOI: https://doi.org/10.1038/nature11475
2. Chen J, Ni J, Xu H, et al. Mechanism and research progress of hydrogen spillover in hydrogen evolution reaction. Journal of Alloys and Compounds. 2024; 1004. doi: 10.1016/j.jallcom.2024.175883 DOI: https://doi.org/10.1016/j.jallcom.2024.175883
3. Wang S, Hu S, Xiang C, et al. Nano-flower-shaped Ru-NiFeAl-LDHs@rGO for efficient hydrogen evolution reaction and oxygen evolution reaction. Journal of Alloys and Compounds. 2025; 1010. doi: 10.1016/j.jallcom.2024.177588 DOI: https://doi.org/10.1016/j.jallcom.2024.177588
4. Xu L, Song Z, Chen H, et al. Recent progress of MoS2 for photocatalytic and electrocatalytic hydrogen generation—A review. Clean Energy Science and Technology. 2024; 2(3): 157. doi: 10.18686/cest.v2i3.157 DOI: https://doi.org/10.18686/cest.v2i3.157
5. Zhang E, Liu J, Ji M, et al. Hollow anisotropic semiconductor nanoprisms with highly crystalline frameworks for high-efficiency photoelectrochemical water splitting. Journal of Materials Chemistry A. 2019; 7(14): 8061-8072. doi: 10.1039/c9ta00925f DOI: https://doi.org/10.1039/C9TA00925F
6. Song K, Bao F, Wang Z, et al. Modulation of RuO2 nanocrystals with facile annealing method for enhancing the electrocatalytic activity on overall water splitting in acid solution. Advanced Science. 2025; 12(9). doi: 10.1002/advs.202409249 DOI: https://doi.org/10.1002/advs.202409249
7. Guo J, Zheng Y, Hu Z, et al. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nature Energy. 2023; 8(3): 264-272. doi: 10.1038/s41560-023-01195-x. DOI: https://doi.org/10.1038/s41560-023-01195-x
8. Dresp S, Dionigi F, Klingenhof M, et al. Direct electrolytic splitting of seawater: opportunities and challenges. ACS Energy Letters. 2019; 4(4): 933-942. doi: 10.1021/acsenergylett.9b00220 DOI: https://doi.org/10.1021/acsenergylett.9b00220
9. Xie H, Zhao Z, Liu T, et al. A membrane-based seawater electrolyser for hydrogen generation. Nature. 2022; 612(7941): 673-678. doi: 10.1038/s41586-022-05379-5 DOI: https://doi.org/10.1038/s41586-022-05379-5
10. Liu T, Zhao Z, Tang W, et al. In-situ direct seawater electrolysis using floating platform in ocean with uncontrollable wave motion. Nature Communications. 2024; 15(1): 5305. doi: 10.1038/s41467-024-49639-6 DOI: https://doi.org/10.1038/s41467-024-49639-6
11. Liu Y, Wang Y, Fornasiero P, et al. Long‐term durability of seawater electrolysis for hydrogen: from catalysts to systems. Angewandte Chemie International Edition. 2024; 63(47). doi: 10.1002/anie.202412087 DOI: https://doi.org/10.1002/anie.202412087
12. Zhang S, Wang Y, Li S, et al. Concerning the stability of seawater electrolysis: a corrosion mechanism study of halide on Ni-based anode. Nature Communications. 2023; 14(1): 4822. doi: 10.1038/s41467-023-40563-9 DOI: https://doi.org/10.1038/s41467-023-40563-9
13. Zhang S, Xu W, Chen H, et al. Progress in anode stability improvement for seawater electrolysis to produce hydrogen. Advanced Materials. 2024; 36(37): 2311322. doi: 10.1002/adma.202311322 DOI: https://doi.org/10.1002/adma.202311322
14. Liu Q, Yan Z, Gao J, et al. Optimizing platinum location on nickel hydroxide nanosheets to accelerate the hydrogen evolution reaction. ACS Applied Materials & Interfaces. 2020; 12(22): 24683-24692. doi: 10.1021/acsami.0c00534 DOI: https://doi.org/10.1021/acsami.0c00534
15. Wu H, Zhang Q, Liu Q, et al. Research on NiFe - based oxygen evolution reaction catalysts and application in electrolytic seawater hydrogen production (Chinese). Journal of Functional Materials and Devices. 2024; 5. doi: 10.20027/j.gncq.2024.0036
16. Lokesh S, Srivastava R. Advanced two-dimensional materials for green hydrogen generation: strategies toward corrosion resistance seawater electrolysis─review and future perspectives. Energy & Fuels. 2022; 36(22): 13417-13450. doi: 10.1021/acs.energyfuels.2c02013 DOI: https://doi.org/10.1021/acs.energyfuels.2c02013
17. Zhao C, Ding Z, Zhang K, et al. Comprehensive chlorine suppression: advances in materials and system technologies for direct seawater electrolysis. Nano-Micro Letters. 2025; 17(1): 113. doi: 10.1007/s40820-025-01653-z DOI: https://doi.org/10.1007/s40820-025-01653-z
18. He D, Yang P, Yang K, et al. Durable seawater electrolysis enabled by chloride rejection on hydroxide trapping anode. Journal of Energy Chemistry. 2025; 107: 407-415. doi: 10.1016/j.jechem.2025.03.063 DOI: https://doi.org/10.1016/j.jechem.2025.03.063
19. Ren J, Chen L, Yuan Z. Electrocatalytic seawater splitting from direct electrolysis to hybrid electrolysis: challenges and opportunities. Materials Today. 2025; 86: 282-316. doi: 10.1016/j.mattod.2025.03.003 DOI: https://doi.org/10.1016/j.mattod.2025.03.003
20. Du H, Sun T, Wang M, et al. Impact of harmful ions in seawater on electrolysis catalysts: challenges and mitigation strategies. Chemical Communications. 2025; 61(31): 5719-5730. doi: 10.1039/d5cc00844a DOI: https://doi.org/10.1039/D5CC00844A
21. Huang J, Jiang Y, An T, et al. Increasing the active sites and intrinsic activity of transition metal chalcogenide electrocatalysts for enhanced water splitting. Journal of Materials Chemistry A. 2020; 8(48): 25465-25498. doi: 10.1039/d0ta08802a DOI: https://doi.org/10.1039/D0TA08802A
22. Lim A, Ham K, Elrefaei S, et al. Operando interpretation of reaction mechanisms and local phenomena on OER catalysts in seawater electrolysis. Current Opinion in Electrochemistry. 2024; 47: 101560. doi: 10.1016/j.coelec.2024.101560 DOI: https://doi.org/10.1016/j.coelec.2024.101560
23. Xu W, Wang H. Earth-abundant amorphous catalysts for electrolysis of water. Chinese Journal of Catalysis. 2017; 38(6): 991-1005. doi: 10.1016/s1872-2067(17)62810-9 DOI: https://doi.org/10.1016/S1872-2067(17)62810-9
24. Burke MS, Enman LJ, Batchellor AS, et al. Oxygen evolution reaction electrocatalysis on transition metal oxides and (oxy)hydroxides: activity trends and design principles. Chemistry of Materials. 2015; 27(22): 7549-7558. doi: 10.1021/acs.chemmater.5b03148 DOI: https://doi.org/10.1021/acs.chemmater.5b03148
25. Chen L, Wang H, Tan L, et al. PEO-PPO-PEO induced holey NiFe-LDH nanosheets on Ni foam for efficient overall water-splitting and urea electrolysis. Journal of Colloid and Interface Science. 2022; 618: 141-148. doi: 10.1016/j.jcis.2022.03.072 DOI: https://doi.org/10.1016/j.jcis.2022.03.072
26. Song Y, Jiang G, Chen Y, et al. Effects of chloride ions on corrosion of ductile iron and carbon steel in soil environments. Scientific Reports. 2017; 7(1): 6865. doi: 10.1038/s41598-017-07245-1 DOI: https://doi.org/10.1038/s41598-017-07245-1
27. Niu HJ, Ran N, Zhou W, et al. Synergistic atomic environment optimization of nickel–iron dual sites by Co doping and Cr vacancy for electrocatalytic oxygen evolution. Journal of the American Chemical Society. 2025; 147(3): 2607-2615. doi: 10.1021/jacs.4c14675 DOI: https://doi.org/10.1021/jacs.4c14675
28. Du J, Li Z, Wang L, et al. Anion exchange membrane seawater electrolysis at 1.0 A cm−2 with an anode catalyst stable for 9000 H. Advanced Science. 2025; 2416661. doi: 10.1002/advs.202416661 DOI: https://doi.org/10.1002/advs.202416661
29. Yu J, Zhang Y, Zhang N, et al. The interface engineering strategy assists the 3D core-shell structure Co3S4/CuS@NiFe LDH nanocoral spheres to achieve significant overall water splitting (Chinese). Chinese Chemical Letters. 2025; 110830. doi: 10.1016/j.cclet.2025.110830 DOI: https://doi.org/10.1016/j.cclet.2025.110830
30. Jing C, Dong B, Zhang Y. Chemical modifications of layered double hydroxides in the supercapacitor. Energy & environmental materials. 2020; 3(3): 346-379. doi: 10.1002/eem2.12116 DOI: https://doi.org/10.1002/eem2.12116
31. Cai J, He X, Dong Q, et al. Employing shielding effect of intercalated cinnamate anion in NiFe LDH for stable and efficient seawater oxidation. Surfaces and Interfaces. 2024; 51: 104772. doi: 10.1016/j.surfin.2024.104772 DOI: https://doi.org/10.1016/j.surfin.2024.104772
32. Song L, Chi J, Tang J, et al. Anode design principles for efficient seawater electrolysis and inhibition of chloride oxidation (Chinese). Chinese Journal of Catalysis. 2024; 66: 53-75. doi: 10.1016/s1872-2067(24)60126-9 DOI: https://doi.org/10.1016/S1872-2067(24)60126-9
33. Cai Z, Liang J, Li Z, et al. Stabilizing NiFe sites by high-dispersity of nanosized and anionic Cr species toward durable seawater oxidation. Nature Communications. 2024; 15(1): 6624. doi: 10.1038/s41467-024-51130-1 DOI: https://doi.org/10.1038/s41467-024-51130-1
34. Kuang Y, Kenney MJ, Meng Y, et al. Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels. Proceedings of the National Academy of Sciences. 2019; 116(14): 6624-6629. doi: 10.1073/pnas.1900556116 DOI: https://doi.org/10.1073/pnas.1900556116
35. Hung WH, Xue BY, Lin TM, et al. A highly active selenized nickel–iron electrode with layered double hydroxide for electrocatalytic water splitting in saline electrolyte. Materials Today Energy. 2021; 19: 100575. doi: 10.1016/j.mtener.2020.100575 DOI: https://doi.org/10.1016/j.mtener.2020.100575
36. Chen H, Liu P, Li W, et al. Stable seawater electrolysis over 10 000 H via chemical fixation of sulfate on NiFeBa‐LDH. Advanced Materials. 2024; 36(45): 2411302. doi: 10.1002/adma.202411302 DOI: https://doi.org/10.1002/adma.202411302
37. Ma T, Xu W, Li B, et al. The critical role of additive sulfate for stable alkaline seawater oxidation on nickel‐based electrodes. Angewandte Chemie International Edition. 2021; 60(42): 22740-22744. doi: 10.1002/anie.202110355 DOI: https://doi.org/10.1002/anie.202110355
38. Huang C, Wang Z, Cheng S, et al. Challenges and strategies of chlorine inhibition in anode systems for seawater electrolysis. Science China Chemistry. 2024; 67(10): 3198-3208. doi: 10.1007/s11426-024-2121-0 DOI: https://doi.org/10.1007/s11426-024-2121-0
39. Huang C, Zhou J, Duan D, et al. Roles of heteroatoms in electrocatalysts for alkaline water splitting: A review focusing on the reaction mechanism (Chinese). Chinese Journal of Catalysis. 2022; 43(8): 2091-2110. DOI: https://doi.org/10.1016/S1872-2067(21)64052-4
40. Chen S, Zhuo Y, Wang X, et al. Advances of layered double hydroxide electrocatalysts for high-current-density alkaline water/seawater splitting. Coordination Chemistry Reviews. 2024; 510: 215832. doi: 10.1016/j.ccr.2024.215832 DOI: https://doi.org/10.1016/j.ccr.2024.215832
41. Zhang Z, Ye K, Du H, et al. In situ electrodeposition synthesis of CoP@NiFe LDH heterostructure as high-performance electrocatalyst for enhanced seawater electrolysis. International Journal of Hydrogen Energy. 2024; 62: 722-731. doi: 10.1016/j.ijhydene.2024.02.202 DOI: https://doi.org/10.1016/j.ijhydene.2024.02.202
42. Ge S, Shen X, Gao J, et al. Synergy of Mo doping and heterostructures in FeCo2S4@Mo-NiCo LDH/NF as durable and corrosion-resistance bifunctional electrocatalyst towards seawater electrolysis at industrial current density. Chemical Engineering Journal. 2024; 485: 150161. doi: 10.1016/j.cej.2024.150161 DOI: https://doi.org/10.1016/j.cej.2024.150161
43. Yu L, Xiao J, Huang C, et al. High-performance seawater oxidation by a homogeneous multimetallic layered double hydroxide electrocatalyst. Proceedings of the National Academy of Sciences. 2022; 119(18). doi: 10.1073/pnas.2202382119 DOI: https://doi.org/10.1073/pnas.2202382119
44. Gupta A, Sadhanala HK, Gedanken A. Iron doped cobalt nickel layered double hydroxide supported on nickel foam as a robust electrocatalyst for highly efficient water oxidation in alkaline sea water. Electrochimica Acta. 2023; 470: 143269. doi: 10.1016/j.electacta.2023.143269 DOI: https://doi.org/10.1016/j.electacta.2023.143269
45. Pearson RG. Hard and soft acids and bases, HSAB, part II: Underlying theories. Journal of Chemical Education. 1968; 45(10): 643. doi: 10.1021/ed045p643 DOI: https://doi.org/10.1021/ed045p643
46. Tu Q, Liu W, Jiang M, et al. Preferential adsorption of hydroxide ions onto partially crystalline NiFe-layered double hydroxides leads to efficient and selective OER in alkaline seawater. ACS Applied Energy Materials. 2021; 4(5): 4630-4637. doi: 10.1021/acsaem.1c00262 DOI: https://doi.org/10.1021/acsaem.1c00262
47. Guo J, Wang R, Wang Q, et al. Constructing an OH−-enriched microenvironment on the electrode surface for natural seawater electrolysis. Nano Research. 2024; 17(11): 9483-9489. doi: 10.1007/s12274-024-6873-1 DOI: https://doi.org/10.1007/s12274-024-6873-1
48. Zhu J, Cui T, Chi J, et al. Frustrated lewis pair mediated f‐p‐d orbital coupling: achieving selective seawater oxidation and breaking *OH and *OOH scaling relationship. Angewandte Chemie International Edition. 2024; 64(2). doi: 10.1002/anie.202414721 DOI: https://doi.org/10.1002/anie.202414721
49. Guo P, Liu D, Wu R. Recent progress in design strategy of anode for seawater electrolysis. Small Structures. 2023; 4(12): 2300192. doi: 10.1002/sstr.202300192 DOI: https://doi.org/10.1002/sstr.202300192
50. Yoon W, Park YH, Jin X, et al. Interface-engineered hybrid electrocatalysts of Ti@holey-TiN/layered-double-hydroxides for efficient seawater electrolysis. Journal of Materials Chemistry A. 2024; 12(32): 21016-21024. doi: 10.1039/d4ta02886d DOI: https://doi.org/10.1039/D4TA02886D
51. Wang Z, Wang C, Ye L, et al. MnOx film-coated NiFe-LDH nanosheets on Ni foam as selective oxygen evolution electrocatalysts for alkaline seawater oxidation. Inorganic Chemistry. 2022; 61(38): 15256-15265. doi: 10.1021/acs.inorgchem.2c02579 DOI: https://doi.org/10.1021/acs.inorgchem.2c02579
52. Zhou L, Guo D, Wu L, et al. A restricted dynamic surface self-reconstruction toward high-performance of direct seawater oxidation. Nature Communications. 2024; 15(1): 2481. doi: 10.1038/s41467-024-46708-8 DOI: https://doi.org/10.1038/s41467-024-46708-8
53. Jadhav AR, Kumar A, Lee J, et al. Stable complete seawater electrolysis by using interfacial chloride ion blocking layer on catalyst surface. Journal of Materials Chemistry A. 2020; 8(46): 24501-24514. doi: 10.1039/d0ta08543j DOI: https://doi.org/10.1039/D0TA08543J
54. Deng P, Xue R, Lu J, et al. Strategies for designing anti‐chlorine corrosion catalysts in seawater splitting. Advanced Energy Materials. 2025; 15(14): 2405749. doi: 10.1002/aenm.202405749 DOI: https://doi.org/10.1002/aenm.202405749
55. Liu W, Yu J, Li T, et al. Self-protecting CoFeAl-layered double hydroxides enable stable and efficient brine oxidation at 2 A cm−2. Nature Communications. 2024; 15(1): 4712. doi: 10.1038/s41467-024-49195-z DOI: https://doi.org/10.1038/s41467-024-49195-z
56. Zhang F, Liu Y, Wu L, et al. Efficient alkaline seawater oxidation by a three-dimensional core-shell dendritic NiCo@NiFe layered double hydroxide electrode. Materials Today Physics. 2022; 27: 100841. doi: 10.1016/j.mtphys.2022.100841 DOI: https://doi.org/10.1016/j.mtphys.2022.100841
57. Bhardwaj AA, Vos JG, Beatty MES, et al. Ultrathin silicon oxide overlayers enable selective oxygen evolution from acidic and unbuffered pH-neutral seawater. ACS Catalysis. 2021; 11(3): 1316-1330. doi: 10.1021/acscatal.0c04343 DOI: https://doi.org/10.1021/acscatal.0c04343
58. Moon G hee, Lim J, Kim B ju, et al. Perspective on direct seawater electrolysis and electrodesalination: innovations and future directions for mining green X. Green Chemistry. 2025; 27(4): 982-1005. doi: 10.1039/d4gc04930f DOI: https://doi.org/10.1039/D4GC04930F
59. Zhou Q, Liao L, Zhou H, et al. Innovative strategies in design of transition metal-based catalysts for large-current-density alkaline water/seawater electrolysis. Materials Today Physics. 2022; 26: 100727. doi: 10.1016/j.mtphys.2022.100727 DOI: https://doi.org/10.1016/j.mtphys.2022.100727
60. Wang J, Liu Y, Yang G, et al. MXene-Assisted NiFe sulfides for high-performance anion exchange membrane seawater electrolysis. Nature Communications. 2025; 16(1): 1319. doi: 10.1038/s41467-025-56639-7 DOI: https://doi.org/10.1038/s41467-025-56639-7
61. Lv J, Chen Z. Analysis the performance of hydrophilic and corrosion resistant coatings on the distillation desalination tube in high temperature seawater. IOP Conference Series: Earth and Environmental Science. 2021; 945(1): 012043. doi: 10.1088/1755-1315/945/1/012043 DOI: https://doi.org/10.1088/1755-1315/945/1/012043
62. Fan R, Liu C, Li Z, et al. Ultrastable electrocatalytic seawater splitting at ampere-level current density. Nature Sustainability. 2024; 7(2): 158-167. doi: 10.1038/s41893-023-01263-w DOI: https://doi.org/10.1038/s41893-023-01263-w
63. Chen H, Gao R, Chen H, et al. Ruthenium and silver synergetic regulation NiFe LDH boosting long‐duration industrial seawater electrolysis. Advanced Functional Materials. 2024; 34(25): 2315674. doi: 10.1002/adfm.202315674 DOI: https://doi.org/10.1002/adfm.202315674
64. Liu H, Shen W, Jin H, et al. High‐performance alkaline seawater electrolysis with anomalous chloride promoted oxygen evolution reaction. Angewandte Chemie International Edition. 2023; 62(46). doi: 10.1002/anie.202311674 DOI: https://doi.org/10.1002/anie.202311674
65. Zhang Y, Wan W, Peng Y, et al. Corrosion-resistant single-atom catalysts for direct seawater electrolysis. National Science Review. 2025; 12(4). doi: 10.1093/nsr/nwaf060 DOI: https://doi.org/10.1093/nsr/nwaf060
66. Sha Q, Wang S, Yan L, et al. 10,000-h-stable intermittent alkaline seawater electrolysis. Nature. 2025; 639(8054): 360-367. doi: 10.1038/s41586-025-08610-1 DOI: https://doi.org/10.1038/s41586-025-08610-1
67. Jia Y, Xie Y. Progress in the modification of layered bimetallic hydroxides and electrolysis of water. Materials Research and Application. 2024. doi: 10.20038/j.cnki.mra.2024.000027




.jpg)
.jpg)

