Research advances in utilization of CO2 resources for oxygen production in Space Station and Mars environments
DOI:
https://doi.org/10.18686/cest310Keywords:
manned spaceflight; conversion of CO2 to O2; thermal catalysis; electrocatalysis; plasma catalysisAbstract
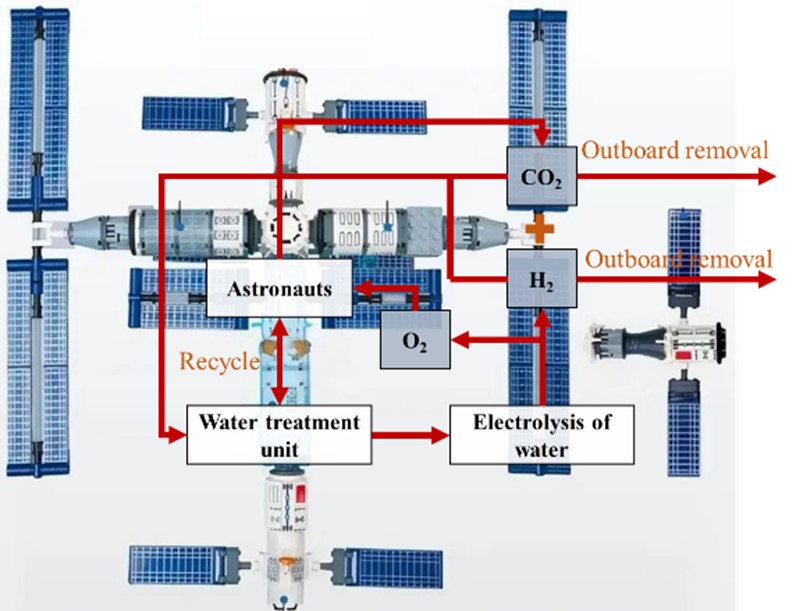
The establishment of stable cycling of CO2 and O2 is essential for Environmental Control and Life Support Systems (ECLSS) in extraterrestrial environments, particularly for long-duration missions aboard Space Stations and future Martian bases. The development of CO2-to-O2 technologies demonstrating superior oxygen recovery rates, enhanced CO2 conversion efficiency, and optimized energy efficiency is critical for achieving closed-loop material regeneration. This review systematically examines technological status in extraterrestrial CO2-to-O2 conversion, categorizing emerging approaches into two frameworks: “two-step oxygen generation” and “one-step oxygen generation”. Two-step oxygen generation includes thermal catalytic CO2 hydrogenation reduction and electrolysis of water for O2 production, which are primarily utilized in Space Station; one-step oxygen generation encompasses electrocatalytic reduction of CO2 and plasma catalytic CO2 conversion, which are predominantly employed in Martian environments. Through comparative analysis of underlying principles and operational characteristics, we identify three critical challenges impeding technological maturation: (1) The deactivation of catalytic materials, the formation of carbon deposits, and the inadequacy of catalytic mechanisms; (2) the description of the transformation process is unclear, making it challenging to regulate the conversion. Additionally, suppressing side reactions proves to be difficult; and (3) the degree of recycling for a single technological substance is relatively low. The development of effective, efficient, stable, and reliable CO2-to-O2 technology will provide a solid foundation for reducing launch costs and ensuring sustainable human habitation in extraterrestrial environments.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Author(s)

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
1. Wu Z, Gao F, Deng Y, et al. Key Technology Review of Research on Regenerative Environmental Control and Life Support System for Space Station (Chinese). Space Medicine & Medical Engineering. 2018; 31(2): 105–111.
2. Hou Q. Research Status and Development Trend of Environmental Control and Life Support Subsystem on Foreign Space Stations (Chinese). Space International. 2015; (1): 51–57.
3. Li X. A Review of Foreign Patent Technologies for Regenerative Life Support Systems in Space Stations (Chinese). Science & Technology Vision. 2024; 14(19): 1–4.
4. Tang L, Gao F, Deng Y, et al. Research on Environmental Control and Life Support System (ECLSS) of China’s Manned Spacecraft (Chinese). Space Medicine & Medical Engineering. 2008; (3): 167–174.
5. Wang K, Gao F. Review and Prospects of Fifty Years’ Development of Environment Control and Life Support System in Manned Spacecraft (Chinese). Space Medicine & Medical Engineering. 2011; 24(6): 435–443.
6. Ash RL, Dowler WL, Varsi G. Feasibility of Rocket Propellant Production on Mars. Acta Astronautica. 1978; 5(9): 705–724. DOI: https://doi.org/10.1016/0094-5765(78)90049-8
7. Ouyang Z, Xiao F. The Mars and its environment (Chinese). Spacecraft Environment Engineering. 2012; 29(6): 591–601.
8. Forsythe RK, Verostko CE, Cusick RJ, et al. A study of Sabatier reactor operation in zero ‘G’. In: Proceedings of the 14th Intersociety Conference on Environmental Systems; 16-19 July 1984; San Diego, California. DOI: https://doi.org/10.4271/840936
9. McKellar M, Stoots C, Sohal M, et al. The Concept and Analytical Investigation of CO2 and Steam Co-Electrolysis for Resource Utilization in Space Exploration. In: Proceedings of the 40th International Conference on Environment Systems; 11–15 July 2010; Barcelona, Spain. DOI: https://doi.org/10.2514/6.2010-6273
10. Strumpf HJ, Chin CY, Lester GR, et al. Sabatier Carbon Dioxide Reduction System for Long-Duration Manned Space Application. In: Proceedings of the 2lst International Conference on Environmental Systems; 15–18 July 1991; San Francisco, CA, USA. pp. 1541–1553. DOI: https://doi.org/10.4271/911541
11. Zhuang Y, Simakov DSA. Autothermal CO2 hydrogenation reactor for renewable natural gas generation: Experimental proof-of-concept. Reaction Chemistry & Engineering. 2022; 7(11): 2285–2297. DOI: https://doi.org/10.1039/D2RE00236A
12. Sakurai M, Shima A, Sone Y, et al. Air Revitalization Demonstration on the JEM (KIBO) for Manned Space Exploration. In: Proceedings of the 43rd International Conference on Environmental Systems; 14–18 July 2013; Vail, CO, USA. DOI: https://doi.org/10.2514/6.2013-3449
13. Crawford JM, Petel BE, Rasmussen MJ, et al. Influence of residual chlorine on Ru/TiO2 active sites during CO2 methanation. Applied Catalysis A-General. 2023; 663. DOI: https://doi.org/10.1016/j.apcata.2023.119292
14. Canales R, Gil-Calvo M, Barrio VL. UV and visible-light photocatalysis using Ni-Co bimetallic and monometallic hydrotalcite-like materials for enhanced CO2 methanation in sabatier reaction. Heliyon. 2023; 9(8): e18456. DOI: https://doi.org/10.1016/j.heliyon.2023.e18456
15. Kruchinin R, Dieguez O. Carbon Dioxide Reduction on Transition Metal Dichalcogenides with Ni and Cu Edge Doping: A Density-Functional Theory Study. ChemPhysChem. 2023; 24(10): e202200765. DOI: https://doi.org/10.1002/cphc.202200765
16. Trevisan SVC, Oliveira LG, de Andrade Schaffner R, et al. Performance of Ni/Si-MCM-41 catalysts in CO2 methanation. Canadian Journal of Chemical Engineering. 2024; 102(8): 2724–2738. DOI: https://doi.org/10.1002/cjce.25224
17. Liao W, Yue M, Chen J, et al. Decoupling the Interfacial Catalysis of CeO2-Supported Rh Catalysts Tuned by CeO2 Morphology and Rh Particle Size in CO2 Hydrogenation. ACS Catalysis. 2023; 13(8): 5767–5779. DOI: https://doi.org/10.1021/acscatal.3c00512
18. Tashiro K, Konno H, Yanagita A, et al. Direct Catalytic Conversion of Carbon Dioxide to Liquid Hydrocarbons over Cobalt Catalyst Supported on Lanthanum (III) Ion-Doped Cerium (IV) Oxide. ChemCatChem. 2024; 16(17): e202400261. DOI: https://doi.org/10.1002/cctc.202400261
19. Molinet-Chinaglia C, Shafiq S, Serp P. Low Temperature Sabatier CO2 Methanation. ChemCatChem. 2024. 16(24): e202401213. DOI: https://doi.org/10.1002/cctc.202401213
20. Khan S, Dai X, Ali T, et al. Recent advances on photo-thermo-catalysis for carbon dioxide methanation. International Journal of Hydrogen Energy. 2023; 48(64): 24756–24787. DOI: https://doi.org/10.1016/j.ijhydene.2022.09.224
21. Ye R, Wang X, Lu Z, et al. Construction of robust Ni-based catalysts for low-temperature Sabatier reaction. Chemical Communications. 2024; 60(81): 11466–11482. DOI: https://doi.org/10.1039/D4CC04342A
22. Sajjadi B, Chen WY. Catalytic non-thermal milli-pulse plasma for methanation of CO2 without carbon deposition and catalyst deactivation. Chemical Engineering Journal. 2023; 469: 143428. DOI: https://doi.org/10.1016/j.cej.2023.143428
23. Hasegawa T, Toko S, Kamataki K, et al. Improving the efficiency of Sabatier reaction through H2O removal with low-pressure plasma catalysis. Japanese Journal of Applied Physics. 2023; 62: 1028. DOI: https://doi.org/10.35848/1347-4065/ace831
24. Luo Y, Huang H, Li C, et al. Highly Efficient and Selective Photothermal Catalytic CO2 Reduction to CH4 Using the CoNi Bimetallic-Modified Gd2O3 &Co3O4 Nanocomposite. ACS Sustainable Chemistry & Engineering. 2024; 12(42): 15682–15695. DOI: https://doi.org/10.1021/acssuschemeng.4c06369
25. Zhu XJ, Zong HB, Perez CJV, et al. Supercharged CO2 Photothermal Catalytic Methanation: High Conversion, Rate, and Selectivity. Angewandte Chemie-International Edition. 2023; 135(22): e202218694. DOI: https://doi.org/10.1002/ange.202218694
26. Holmes RF, King CD, Keller EE. Bosch CO2 Reduction System Development. 1976. Available online: https://ntrs.nasa.gov/citations/19750025653 (accessed on 15 October 2025).
27. Abney M, Mansell JM. The Bosch Process—Performance of a Developmental Reactor and Experimental Evaluation of Alternative Catalysts. In: Proceedings of the 40th International Conference on Environmental Systems; 11–15 July 2010; Barcelona, Spain. pp. 6272–6292. DOI: https://doi.org/10.2514/6.2010-6272
28. Abney MB, Mansell JM, Atkins B, et al. Advanced Oxygen Recovery via Series-Bosch Technology. In: Proceedings of the 45th International Conference on Environmental Systems; 12–16 July 2015; Bellevue, WA, USA. pp. 82–97.
29. Wang J, Wang J, Feng J, et al. Photochemical CO2 hydrogenation to carbon nanotubes and H2O for oxygen recovery in space exploration. Joule. 2024; 8(11): 3126-3141. DOI: https://doi.org/10.1016/j.joule.2024.08.007
30. Xu S, Li H, Yin Z. Analysis of technological upgrade of environmental control and life support system of International Space Station (Chinese). Aerospace China. 2024; (4): 17–24.
31. Samsonov NM, Bobe LS, Gavrilov LI, et al. Water Recovery and Oxygen Generation by Electrolysis Aboard the International Space Station. In: Proceedings of the 32nd International Conference on Environmental Systems; 15–18 July 2002; San Antonio, TX, USA. DOI: https://doi.org/10.4271/2002-01-2358
32. Takada K, Velasquez LE, Van Keuren S, et al. Advanced Oxygen Generation Assembly for Exploration Missions. In: Proceedings of the 49th International Conference on Environmental Systems; 7–11 July 2019; Boston, MA, USA. pp. 2019–2107.
33. Takada K, Hornyak D, Garr J, et al. Status of the Advanced Oxygen Generation Assembly. In: Proceedings of the 52nd International Conference on Environmental Systems; 16–20 July 2023; Calgary, Canada. pp. 2023–2311.
34. Takada K, Ghariani AE, Van Keuren S, et al. Oxygen Generation Assembly Design for Exploration Missions. In: Proceedings of the 48th International Conference on Environmental Systems; 8–12 July 2018; Albuquerque, NM, USA. pp. 2018–2113.
35. Zhang Z, Song L. Hydrogen production by water electrolysis: Advances, challenges and future prospects (Chinese). Chinese Journal of Engineering. 2025; 47(2): 1–15.
36. Huo M, Liu X, Chen X, et al. Research progress on non-precious metal catalysts for hydrogen production by anion exchange membrane electrolysis (Chinese). Industrial Catalysis. 2024; 32(11): 34–42.
37. Zhao Q, Feng Y, Fu X, et al. Research progress in non-noble metal catalysts for oxygen evolution in anionic membrane water electrolysis (Chinese). Petrochemical Technology. 2024; 53(10): 1491–1496.
38. Khan JB, Liang Y. Recent Progress in Non-Noble Metal Catalysts for Oxygen Evolution Reaction: A Focus on Transition and Rare-Earth Elements. Chemical Record. 2024; 24(12): e202400151. DOI: https://doi.org/10.1002/tcr.202400151
39. Kwon CY, Jeong JY, Yang J, et al. Effect of Copper Cobalt Oxide Composition on Oxygen Evolution Electrocatalysts for Anion Exchange Membrane Water Electrolysis. Frontiers in Chemistry. 2020; 8: 600908. DOI: https://doi.org/10.3389/fchem.2020.600908
40. Jang MJ, Yang J, Lee J, et al. Superior performance and stability of anion exchange membrane water electrolysis: pH-controlled copper cobalt oxide nanoparticles for the oxygen evolution reaction. Journal of Materials Chemistry A. 2020; 8(8): 4290–4299. DOI: https://doi.org/10.1039/C9TA13137J
41. Gupta G, Scott K, Mamlouk M. Performance of polyethylene based radiation grafted anion exchange membrane with polystyrene-b-poly (ethylene/butylene)-b-polystyrene based ionomer using NiCo2O4 catalyst for water electrolysis. Journal of Power Sources. 2018; 375: 387–396. DOI: https://doi.org/10.1016/j.jpowsour.2017.07.026
42. Yamada I, Fujii H, Takamatsu A, et al. Bifunctional Oxygen Reaction Catalysis of Quadruple Manganese Perovskites. Advanced Materials. 2017; 29(4): 1603004. DOI: https://doi.org/10.1002/adma.201603004
43. Miao X, Wu L, Lin Y, et al. The role of oxygen vacancies in water oxidation for perovskite cobalt oxide electrocatalysts: Are more better. Chemical Communications. 2019; 55(10): 1442–1445. DOI: https://doi.org/10.1039/C8CC08817A
44. Li X, Walsh FC, Pletcher D. Nickel based electrocatalysts for oxygen evolution in high current density, alkaline water electrolysers. Physical Chemistry Chemical Physics. 2011; 13(3): 1162–1167. DOI: https://doi.org/10.1039/C0CP00993H
45. Perez-Alonso FJ, Adan C, Rojas S, et al. Ni/Fe electrodes prepared by electrodeposition method over different substrates for oxygen evolution reaction in alkaline medium. International Journal of Hydrogen Energy. 2014; 39(10): 5204–5212. DOI: https://doi.org/10.1016/j.ijhydene.2013.12.186
46. Lin X, Li X, Shi L, et al. In Situ Electrochemical Restructuring B-Doped Metal-Organic Frameworks as Efficient OER Electrocatalysts for Stable Anion Exchange Membrane Water Electrolysis. Small. 2024; 20(22): 2308517. DOI: https://doi.org/10.1002/smll.202308517
47. Koshikawa H, Murase H, Hayashi T, et al. Single Nanometer-Sized NiFe-Layered Double Hydroxides as Anode Catalyst in Anion Exchange Membrane Water Electrolysis Cell with Energy Conversion Efficiency of 74.7% at 1.0 A cm−2. ACS Catalysis. 2020; 10(3): 1886–1893. DOI: https://doi.org/10.1021/acscatal.9b04505
48. Guo DD, Yu HM, Chi J. Self-Supporting NiFe LDHs@Co-OH-CO3 Nanorod Array Electrode for Alkaline Anion Exchange Membrane Water Electrolyzer. Journal of Electrochemistry. 2022; 28(9): 2214003.
49. Stancati ML, Niehoff JC, Wells WC, et al. In situ propellant production—A new potential for round-trip spacecraft. In: Proceedings of the Conference on Advanced Technology for Future Space Systems; 8–10 May 1979; Hampton, VA, USA.
50. Kaplan D, Baird R, Flynn H, et al. The 2001 Mars In-situ-propellant-production Precursor (MIP) Flight Demonstration—Project objectives and qualification test results. In: Proceedings of the Space 2000 Conference and Exposition; 19–21 September 2000; Long Beach, CA, USA. DOI: https://doi.org/10.2514/6.2000-5145
51. Hecht M, Hoffman J, Rapp D, et al. Mars Oxygen ISRU Experiment (MOXIE). Space Science Reviews. 2021; 217: 1-76. DOI: https://doi.org/10.1007/s11214-020-00782-8
52. Tao G, Sridhar KR, Chan CL. Study of carbon dioxide electrolysis at electrode/electrolyte interface: Part I. Pt/YSZ interface. Solid State Ionics. 2004; 175(1–4): 615–619. DOI: https://doi.org/10.1016/j.ssi.2004.01.077
53. Wang S, Inoishi A, Hong J, et al. Ni-Fe bimetallic cathodes for intermediate temperature CO2 electrolyzers using a La0.9Sr0.1Ga0.8Mg0.2O3 electrolyte. Journal Of Materials Chemistry A. 2013; 1(40): 12455–12461. DOI: https://doi.org/10.1039/c3ta11863k
54. Jiang Y, Chen F, Xia C. A review on cathode processes and materials for electro-reduction of carbon dioxide in solid oxide electrolysis cells. Journal of Power Sources. 2021; 493: 229713. DOI: https://doi.org/10.1016/j.jpowsour.2021.229713
55. Mogensen M, Skaarup S. Kinetic and geometric aspects of solid oxide fuel cell electrodes. Solid State Ionics. 1996; 86–88(Part 2): 1151–1160. DOI: https://doi.org/10.1016/0167-2738(96)00280-9
56. Rabuni MF, Vatcharasuwan N, Li T, et al. High performance micro-monolithic reversible solid oxide electrochemical reactor. Journal of Power Sources. 2020; 458: 228026. DOI: https://doi.org/10.1016/j.jpowsour.2020.228026
57. Zheng M, Wang S, Yang Y, et al. Barium carbonate as a synergistic catalyst for the H2O/CO2 reduction reaction at Ni-yttria stabilized zirconia cathodes for solid oxide electrolysis cells. Journal of Materials Chemistry A. 2018; 6(6): 2721–2729. DOI: https://doi.org/10.1039/C7TA08249E
58. Kumari N, Haider MA, Tiwari PK, et al. Carbon dioxide reduction on the composite of copper and praseodymium-doped ceria electrode in a solid oxide electrolysis cells. Ionics. 2019; 25(7): 3165–3177. DOI: https://doi.org/10.1007/s11581-019-02837-5
59. Song Y, Min J, Guo Y, et al. Surface Activation by Single Ru Atoms for Enhanced High -Temperature CO2 Electrolysis. Angewandte Chemie International Edition. 2024; 63(5): e202313361. DOI: https://doi.org/10.1002/anie.202313361
60. Tao Y, Ebbesen SD, Mogensen MB. Degradation of solid oxide cells during co-electrolysis of steam and carbon dioxide at high current densities. Journal of Power Sources. 2016; 331: 569. DOI: https://doi.org/10.1016/j.jpowsour.2016.09.074
61. Yan J, Chen H, Dogdibegovic E, et al. High-efficiency intermediate temperature solid oxide electrolyzer cells for the conversion of carbon dioxide to fuels. Journal of Power Sources. 2014; 252: 79–84. DOI: https://doi.org/10.1016/j.jpowsour.2013.11.047
62. He X, Huang X, Sun H, et al. Enhanced CO2 electrolysis with in situ exsolved nanoparticles in the perovskite cathode. New Journal of Chemistry. 2024; 48(13): 5834–5839. DOI: https://doi.org/10.1039/D4NJ00406J
63. He S, He X, Gan L. In situ exsolved Fe nanoparticles enhance the catalytic performance of perovskite cathode materials in solid oxide electrolytic cells. New Journal of Chemistry. 2024; 48(44): 18739–18745. DOI: https://doi.org/10.1039/D4NJ03794D
64. Gao X, Ye L, Xie K. In situ exsolved Ni-Cu alloy nanoparticles for optimization of perovskite electrodes in solid oxide electrolysis cell. Fuel. 2024; 371: 131959. DOI: https://doi.org/10.1016/j.fuel.2024.131959
65. Gao X, Ye L, Xie K. Voltage-driven reduction method to optimize in-situ exsolution of Fe nanoparticles at Sr2Fe1.5+xMo0.5O6 interface. Journal of Power Sources. 2023; 561: 232740. DOI: https://doi.org/10.1016/j.jpowsour.2023.232740
66. Qiu P, Li C, Liu B, et al. Materials of solid oxide electrolysis cells for H2O and CO2 electrolysis: A review. Journal of Advanced Ceramics. 2023; 12(8): 1463–1510. DOI: https://doi.org/10.26599/JAC.2023.9220767
67. Kwon OH, Choi GM. Electrical conductivity of thick film YSZ. Solid State Ionics. 2006; 177(35–36): 3057–3062. DOI: https://doi.org/10.1016/j.ssi.2006.07.039
68. Shi H, Su C, Ran R, et al. Electrolyte materials for intermediate-temperature solid oxide fuel cells. Progress in Natural Science-Materials International. 2020; 30(6): 764–774. DOI: https://doi.org/10.1016/j.pnsc.2020.09.003
69. Li Y, Ye L, Xie K. Enhanced Carbon Dioxide Electrolysis in Zr-Doped Ceria. Energy & Fuels. 2024; 38(18): 18018–18025. DOI: https://doi.org/10.1021/acs.energyfuels.4c03350
70. Jung DW, Lee KT, Wachsman ED. Terbium and Tungsten Co-doped Bismuth Oxide Electrolytes for Low Temperature Solid Oxide Fuel Cells. Journal of the Korean Ceramic Society. 2014; 51(4): 260–264. DOI: https://doi.org/10.4191/kcers.2014.51.4.260
71. Aguadero A, Fawcett L, Taub S, et al. Materials development for intermediate-temperature solid oxide electrochemical devices. Journal of Materials Science. 2012; 47(9): 3925–3948. DOI: https://doi.org/10.1007/s10853-011-6213-1
72. Peng X, Tian Y, Liu Y, et al. An efficient symmetrical solid oxide electrolysis cell with LSFM-based electrodes for direct electrolysis of pure CO2. Journal of CO2 Utilization. 2020; 36: 18–24. DOI: https://doi.org/10.1016/j.jcou.2019.10.017
73. Song Y, Zhang X, Zhou Y, et al. Promoting oxygen evolution reaction by RuO2 nanoparticles in solid oxide CO2 electrolyzer. Energy Storage Materials. 2018; 13: 207–214. DOI: https://doi.org/10.1016/j.ensm.2018.01.013
74. Feng WC, Yu JC, Yang YL, et al. Regulating the High Entropy Component of Double Perovskite for High-Temperature Oxygen Evolution Reaction. ACTA Physico-Chimica Sinica. 2024; 40(6): 2306013. DOI: https://doi.org/10.3866/PKU.WHXB202306013
75. Zhang B, Zhang S, Zhang Z, et al. Metal-supported solid oxide electrolysis cell for direct CO2 electrolysis using stainless steel based cathode. Journal of Power Sources. 2023; 556: 232467. DOI: https://doi.org/10.1016/j.jpowsour.2022.232467
76. Feng D, Zhang C, Jiang WJ, et al. Design and trial of extraterrestrial artificial photosynthesis device (Chinese). Chinese Space Science and Technology. 2020; 40(6): 13–22. DOI: https://doi.org/10.11728/cjss2020.01.072
77. George A, Shen B, Craven M, et al. A Review of Non-Thermal Plasma Technology: A novel solution for CO2 conversion and utilization. Renewable & Sustainable Energy Reviews. 2021; 135: 109702. DOI: https://doi.org/10.1016/j.rser.2020.109702
78. Ashford B, Tu X. Non-thermal plasma technology for the conversion of CO2. Current Opinion in Green and Sustainable Chemistry. 2017; 3: 45–49. DOI: https://doi.org/10.1016/j.cogsc.2016.12.001
79. Bogaerts A, Neyts EC. Plasma Technology: An Emerging Technology for Energy Storage. ACS Energy Letters. 2018; 3(4): 1013–1027. DOI: https://doi.org/10.1021/acsenergylett.8b00184
80. Outlaw RA. O2 and CO2 glow-discharge-assisted oxygen transport through Ag. Journal of Applied Physics. 1990; 68(3): 1002–1004. DOI: https://doi.org/10.1063/1.346734
81. Ash RL, Wu D, Outlaw RA. A Study of Glow-Discharge and Permeation Techniques for Extraterrestrial Oxygen Beneficiation. Astronomy and Space Science from The Moon. 1994; 14(6): 259–263. DOI: https://doi.org/10.1016/0273-1177(94)90036-1
82. Wu D, Outlaw RA, Ash RL. Extraction of oxygen from CO2 using glow-discharge and permeation techniques. Journal of Vacuum Science & Technology a-Vacuum Surfaces and Films. 1996; 14(2): 408–414. DOI: https://doi.org/10.1116/1.580098
83. Shi Z, Wu D, Ash RL. An Investigation of Radio Frequency Enhanced Glow Discharge Production of Oxygen. In: Proceedings of the 26th International Conference on Environmental Systems; 8–11 July 1996; Monterey, CA, USA. DOI: https://doi.org/10.4271/961598
84. VuSkoviC L, Ash RL, Shi Z, et al. Radio-Frequency-Discharge Reaction Cell for Oxygen Extraction from Martian Atmosphere. In: Proceedings of the 27th International Conference on Environmental Systems; 14–17 July 1997; Lake Tahoe, NV, USA. DOI: https://doi.org/10.4271/972499
85. Zhang T, Wang X, Zhang Y. Numerical study on simplified reaction set of ground state species in CO2 discharges under Martian atmospheric conditions. Acta Physica Sinica. 2021; 70(21): 228–247. DOI: https://doi.org/10.7498/aps.70.20210664
86. Capezzuto P, Cramarossa F, D’Agostino R, et al. Contribution of vibrational excitation to the rate of carbon dioxide dissociation in electrical discharges. Journal of Physical Chemistry. 1976; 80(8): 882–888. DOI: https://doi.org/10.1021/j100549a024
87. Ogloblina P, Morillo-Candas AS, Silva AF, et al. Mars in situ oxygen and propellant production by non-equilibrium plasmas. Plasma Sources Science & Technology. 2021; 30(6): 065005. DOI: https://doi.org/10.1088/1361-6595/abec28
88. Wang WZ, Berthelot A, Kolev S, et al. CO2 conversion in a gliding arc plasma: 1D cylindrical discharge model. Plasma Sources Science & Technology. 2016; 25(6): 065012. DOI: https://doi.org/10.1088/0963-0252/25/6/065012
89. Wang WZ, Bogaerts A. Effective ionisation coefficients and critical breakdown electric field of CO2 at elevated temperature: Effect of excited states and ion kinetics. Plasma Sources Science & Technology. 2016; 25(5): 055025. DOI: https://doi.org/10.1088/0963-0252/25/5/055025
90. Wang W, Mei D, Tu X, et al. Gliding arc plasma for CO2 conversion: Better insights by a combined experimental and modelling approach. Chemical Engineering Journal. 2017; 330: 11–25. DOI: https://doi.org/10.1016/j.cej.2017.07.133
91. Pietanza LD, Guaitella O, Aquilanti V, et al. Advances in non-equilibrium CO2 plasma kinetics: A theoretical and experimental review. European Physical Journal D. 2021; 75(9): 237. DOI: https://doi.org/10.1140/epjd/s10053-021-00226-0
92. Guerra V, Silva T, Ogloblina P, et al. The case for in situ resource utilisation for oxygen production on Mars by nonequilibrium plasmas. Plasma Sources Science & Technology. 2017; 26(11): 11LT01. DOI: https://doi.org/10.1088/1361-6595/aa8dcc
93. Wang X, Gao S, Zhang Y. Frequency Effects on the Vibrational States and Conversion of CO2 in Radio Frequency Discharges Under Martian Pressure. IEEE Transactions on Plasma Science. 2023; 51(1): 49–59. DOI: https://doi.org/10.1109/TPS.2022.3225240
94. Duan X, Li Y, Ge W, et al. Degradation of CO2 through dielectric barrier discharge microplasma. Greenhouse Gases-Science and Technology. 2015; 5(2): 131–140. DOI: https://doi.org/10.1002/ghg.1425
95. Mei D, Zhu X, Wu C, et al. Plasma-photocatalytic conversion of CO2 at low temperatures: Understanding the synergistic effect of plasma-catalysis. Applied Catalysis B-Environmental. 2016; 182: 525–532. DOI: https://doi.org/10.1016/j.apcatb.2015.09.052
96. Snoeckx R, Zeng YX, Tu X, et al. Plasma-based dry reforming: Improving the conversion and energy efficiency in a dielectric barrier discharge. RSC Advances. 2015; 5(38): 29799–29808. DOI: https://doi.org/10.1039/C5RA01100K
97. Aerts R, Somers W, Bogaerts A. Carbon Dioxide Splitting in a Dielectric Barrier Discharge Plasma: A Combined Experimental and Computational Study. Chemsuschem. 2015; 8(4): 702–716. DOI: https://doi.org/10.1002/cssc.201402818
98. Wang C, Fu Q, Chang Z, et al. Investigation on the products distribution, reaction pathway, and discharge mechanism of low-pressure CO2 discharge by employing a 1D simulation model. Plasma Processes and Polymers. 2021; 18(6): 2000228. DOI: https://doi.org/10.1002/ppap.202000228
99. Fu Q, Wang Y, Chang Z. Study on the conversion mechanism of CO2 to O2 in pulse voltage dielectric barrier discharge at Martian pressure. Journal of CO2 Utilization. 2023; 70: 102430. DOI: https://doi.org/10.1016/j.jcou.2023.102430
100. Fu Q, Ye Z, Guo H, et al. Generation and migration of CO in CO2 DBD glow plasma under Martian pressure. Plasma Processes and Polymers. 2024; 21(11): e2400085. DOI: https://doi.org/10.1002/ppap.202400085
101. Wang XC, Bai JX, Zhang TH, et al. Comprehensive study on plasma chemistry and products in CO2 pulsed discharges under Martian pressure. Vacuum. 2022; 203: 111200. DOI: https://doi.org/10.1016/j.vacuum.2022.111200
102. Ashford B, Wang Y, Wang L, et al. Plasma-Catalytic Conversion of Carbon Dioxide. In: Tu X, Whitehead JC, Nozaki T (editors). Plasma Catalysis: Fundamentals and Applications. Springer Cham; 2019. Volume 106. pp. 271–307. DOI: https://doi.org/10.1007/978-3-030-05189-1_9
103. Snoeckx R, Bogaerts A. Plasma technology—a novel solution for CO2 conversion. Chemical Society Reviews. 2017; 46(19): 5805–5863. DOI: https://doi.org/10.1039/C6CS00066E
104. Sun SR, Wang HX, Mei DH, et al. CO2 conversion in a gliding arc plasma: Performance improvement based on chemical reaction modeling. Journal of CO2 Utilization. 2017; 17: 220–234. DOI: https://doi.org/10.1016/j.jcou.2016.12.009
105. Devid EJ, Ronda-Lloret M, Zhang D, et al. Enhancing CO2 plasma conversion using metal grid catalysts. Journal of Applied Physics. 2021; 129(5): 053306. DOI: https://doi.org/10.1063/5.0033212
106. Kelly S, Mercer E, Gorbanev Y, et al. Plasma-based conversion of Martian atmosphere into life-sustaining chemicals: The benefits of utilizing martian ambient pressure. Journal of CO2 Utilization. 2024; 80: 102668. DOI: https://doi.org/10.1016/j.jcou.2024.102668
107. Fu Q, Ye Z, Wang Y, et al. Effect of Dielectric Barrier Materials on Conversion Characteristics of Low Pressure CO2 Dielectric Barrier Discharge. Acta Petrolei Sinica (Petroleum Processing Section). 2023; 39(5): 1003–1012.
108. Qian M. Study of reaction products and emission spectra of CO2 glow discharge under simulated Mars conditions [Master’s thesis]. Shandong University; 2021.
109. Qian M, Yan F, Zhang P, et al. The Generation of O2 and CO by CO2 Glow Discharge for In-Situ Martian Atmospheric Utilization. Solar System Research. 2024; 58(4): 419–426. DOI: https://doi.org/10.1134/S0038094624700333
110. Fridman A. Plasma Chemistry. Cambridge University Press; 2008. DOI: https://doi.org/10.1017/CBO9780511546075
111. Mei D, Tu X. Conversion of CO2 in a cylindrical dielectric barrier discharge reactor: Effects of plasma processing parameters and reactor design. Journal of CO2 Utilization. 2017; 19: 68–78. DOI: https://doi.org/10.1016/j.jcou.2017.02.015
112. Zhu X, Liu J, Li X, et al. Enhanced effect of plasma on catalytic reduction of CO2 to CO with hydrogen over Au/CeO2 at low temperature. Journal of Energy Chemistry. 2017; 3(26): 488–493. DOI: https://doi.org/10.1016/j.jechem.2016.11.023
113. Nunnally T, Gutsol K, Rabinovich A, et al. Dissociation of CO2 in a low current gliding arc plasmatron. Journal of Physics D-Applied Physics. 2011; 44(27): 274009. DOI: https://doi.org/10.1088/0022-3727/44/27/274009
114. Indarto A, Yang DR, Choi J, et al. Gliding arc plasma processing of CO2 conversion. Journal of Hazardous Materials. 2007; 146(1–2): 309–315. DOI: https://doi.org/10.1016/j.jhazmat.2006.12.023
115. Lazarova S, Paunska T, Vasilev V, et al. Gliding Arc/Glow Discharge for CO2 Conversion: The Role of Discharge Configuration and Gas Channel Thickness. Plasma. 2024; 7(4): 877–890. DOI: https://doi.org/10.3390/plasma7040048
116. Xu Y, Gao Y, Xi D, et al. Spark Discharge Plasma-Enabled CO2 Conversion Sustained by a Compact, Energy-Efficient, and Low-Cost Power Supply. Industrial & Engineering Chemistry Research. 2023; 62(39): 15872–15883. DOI: https://doi.org/10.1021/acs.iecr.3c02393
117. Qiao J, Liu Y, Hong F, et al. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chemical Society Reviews. 2014; 43(2): 631–675. DOI: https://doi.org/10.1039/C3CS60323G
118. Spencer LF, Gallimore AD. Efficiency of CO2 Dissociation in a Radio-Frequency Discharge [J]. Plasma Chemistry and Plasma Processing. 2011; 31(1): 79–89. DOI: https://doi.org/10.1007/s11090-010-9273-0
119. Stewig C, Schuettler S, Urbanietz T, et al. Excitation and dissociation of CO2 heavily diluted in noble gas atmospheric pressure plasma. Journal of Physics D-Applied Physics. 2020; 53(12): 125205. DOI: https://doi.org/10.1088/1361-6463/ab634f
120. Spencer LF, Gallimore AD. CO2 dissociation in an atmospheric pressure plasma/catalyst system: A study of efficiency. Plasma Sources Science & Technology. 2013; 22(1): 015019. DOI: https://doi.org/10.1088/0963-0252/22/1/015019
121. Kiefer CK, Antunes R, Hecimovic A, et al. CO2 dissociation using a lab-scale microwave plasma torch: An experimental study in view of industrial application. Chemical Engineering Journal. 2024; 481: 148326. DOI: https://doi.org/10.2139/ssrn.4584404
122. Chen G, Britun N, Godfroid T, et al. An overview of CO2 conversion in a microwave discharge: The role of plasma-catalysis. Journal of Physics D-Applied Physics. 2017; 50(8): 084001. DOI: https://doi.org/10.1088/1361-6463/aa5616
123. Chen G, Georgieva V, Godfroid T, et al. Plasma assisted catalytic decomposition of CO2. Applied Catalysis B-Environmental. 2016; 190: 115–124. DOI: https://doi.org/10.1016/j.apcatb.2016.03.009
124. Mei D, Zhu X, He Y, et al. Plasma-assisted conversion of CO2 in a dielectric barrier discharge reactor: Understanding the effect of packing materials. Plasma Sources Science & Technology. 2015; 24(1). 015011. DOI: https://doi.org/10.1088/0963-0252/24/1/015011
125. Yap D, Tatibouet JM, Batiot-Dupeyrat C. Carbon dioxide dissociation to carbon monoxide by non-thermal plasma. Journal of CO2 Utilization. 2015; 12: 54–61. DOI: https://doi.org/10.1016/j.jcou.2015.07.002
126. Lu N, Sun D, Zhang C, et al. CO2 conversion in non-thermal plasma and plasma/g-C3N4 catalyst hybrid processes. Journal of Physics D-Applied Physics. 2018; 51(9): 094001. DOI: https://doi.org/10.1088/1361-6463/aaa919
127. Wang K, Ceulemans S, Zhang H, et al. Inhibiting recombination to improve the performance of plasma-based CO2 conversion. Chemical Engineering Journal. 2024; 481: 148648. DOI: https://doi.org/10.1016/j.cej.2024.148684
128. Mei D, Tu X. Atmospheric Pressure Non‐Thermal Plasma Activation of CO2 in a Packed‐Bed Dielectric Barrier Discharge Reactor. ChemPhysChem. 2017; 18(22): 3253–3259. DOI: https://doi.org/10.1002/cphc.201700752
129. Rao MU, Bhargavi KVSS, Chawdhury P, et al. Non-thermal plasma assisted CO2 conversion to CO: Influence of non-catalytic glass packing materials. Chemical Engineering Science. 2023; 267: 118376. DOI: https://doi.org/10.1016/j.ces.2022.118376
130. He L, Yue X, Liu X, et al. Performance of CO2 decomposition in water-cooling DBD plasma reactor. Journal of Physics D-Applied Physics. 2025; 58(10): 105204. DOI: https://doi.org/10.1088/1361-6463/ada2f9
131. Yong T, Zhong H, Pannier E, et al. High-pressure CO2 dissociation with nanosecond pulsed discharges. plasma sources science & technology. 2023; 32(11): 115012. DOI: https://doi.org/10.1088/1361-6595/ad066e
132. Zhu S, Zhou A, Yu F, et al. Enhanced CO2 decomposition via metallic foamed electrode packed in self-cooling DBD plasma device. Plasma Science & Technology. 2019; 21(8): 085504. DOI: https://doi.org/10.1088/2058-6272/ab15e5
133. Lu N, Liu N, Zhang C, et al. CO2 conversion promoted by potassium intercalated g-C3N4 catalyst in DBD plasma system. Chemical Engineering Journal. 2021; 417: 129183. DOI: https://doi.org/10.1016/j.cej.2021.129283
134. Golubev O, Maximov A. Hybrid Plasma-Catalytic CO2 Dissociation over Basic Metal Oxides Combined with CeO2. Processes. 2023; 11(5): 1553. DOI: https://doi.org/10.3390/pr11051553
135. Ashford B, Wang Y, Poh C, et al. Plasma-catalytic conversion of CO2 to CO over binary metal oxide catalysts at low temperatures. Applied Catalysis B-Environmental. 2020; 276: 119110. DOI: https://doi.org/10.1016/j.apcatb.2020.119110
136. Wang L, Du X, Yi Y, et al. Plasma-enhanced direct conversion of CO2 to CO over oxygen-deficient Mo-doped CeO2. Chemical Communications. 2020; 56(94): 14801–14804. DOI: https://doi.org/10.1039/D0CC06514E
137. Hatami H, Khani M, Rad SAR, et al. CO2 conversion in a dielectric barrier discharge plasma by argon dilution over MgO/HKUST-1 catalyst using response surface methodology. Heliyon. 2024; 10(4): e26280. DOI: https://doi.org/10.1016/j.heliyon.2024.e26280




.jpg)
.jpg)

