Transition metal dichalcogenides-based electrocatalysts for green hydrogen production via water electrolysis: Design principles and modulation strategies
DOI:
https://doi.org/10.18686/cest294Keywords:
electrocatalytic water splitting; hydrogen evolution reaction; oxygen evolution reaction; transition metal dichalcogenides; electrocatalytic performanceAbstract
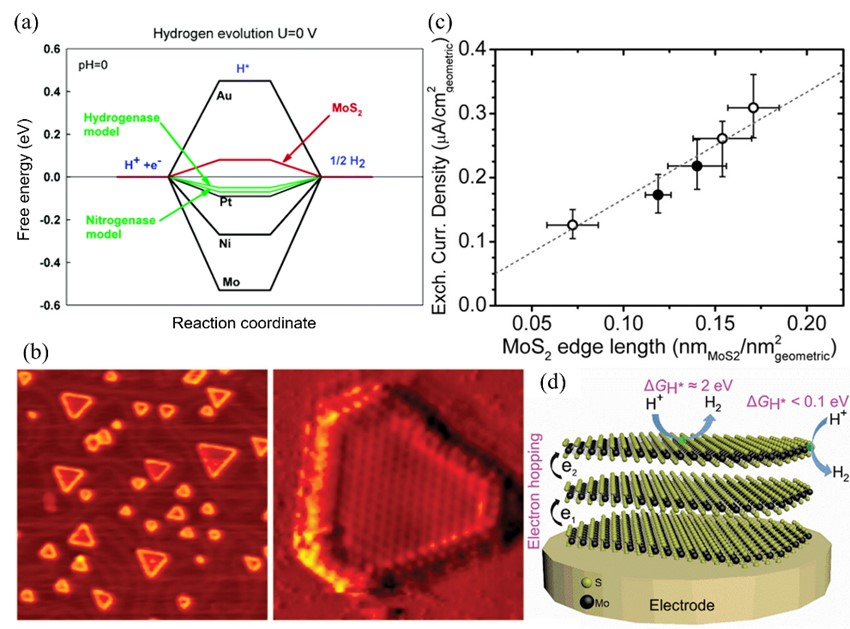
The development of renewable energy-powered water electrolysis technology serves as a crucial prerequisite for realizing the large-scale application of hydrogen economy. Currently, commercial catalysts for water electrolysis predominantly rely on platinum-group noble metals, whose scarcity and exorbitant costs significantly hinder practical implementation of hydrogen production through water splitting. As promising alternatives to noble metal catalysts, transition metal dichalcogenides (TMDs) have attracted considerable research attention due to their high intrinsic catalytic activity and cost-effectiveness. Nevertheless, the catalytic performance of TMDs still lags behind that of noble metal benchmarks, prompting extensive and systematic investigations into performance enhancement and catalytic mechanisms. This review comprehensively summarizes strategic approaches for optimizing the electrocatalytic performance of TMDs in water electrolysis, integrating fundamental reaction principles, rational design philosophies for electrocatalysts, and the structure-property relationships of TMDs. Finally, we provide insightful perspectives on current challenges and future research directions in this rapidly evolving field.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Author(s)

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
1. Kment Š, Bakandritsos A, Tantis I, et al. Single Atom Catalysts Based on Earth-Abundant Metals for Energy-Related Applications. Chemical Reviews. 2024; 124(21): 11767–11847. DOI: https://doi.org/10.1021/acs.chemrev.4c00155
2. Østergaard PA, Duic N, Noorollahi Y, et al. Renewable energy for sustainable development. Renewable Energy. 2022; 199: 1145–1152. DOI: https://doi.org/10.1016/j.renene.2022.09.065
3. Nikolaidis P, Poullikkas A. A comparative overview of hydrogen production processes. Renewable and Sustainable Energy Reviews. 2017; 67: 597–611. DOI: https://doi.org/10.1016/j.rser.2016.09.044
4. Layton BE. A Comparison of Energy Densities of Prevalent Energy Sources in Units of Joules Per Cubic Meter. International Journal of Green Energy. 2008; 5(6): 438–455. DOI: https://doi.org/10.1080/15435070802498036
5. Yukesh Kannah R, S Kavitha, Preethi, et al. Techno-economic assessment of various hydrogen production methods—A review. Bioresource Technology. 2021; 319: 124175. DOI: https://doi.org/10.1016/j.biortech.2020.124175
6. Shiva Kumar S, Lim H. An overview of water electrolysis technologies for green hydrogen production. Energy Reports. 2022; 8: 13793–13813. DOI: https://doi.org/10.1016/j.egyr.2022.10.127
7. Kumar A, Daw P, Milstein D. Homogeneous Catalysis for Sustainable Energy: Hydrogen and Methanol Economies, Fuels from Biomass, and Related Topics. Chemical Reviews. 2022; 122(1): 385–441. DOI: https://doi.org/10.1021/acs.chemrev.1c00412
8. Mosca L, Medrano Jimenez JA, Wassie SA, et al. Process design for green hydrogen production. International Journal of Hydrogen Energy. 2020; 45(12): 7266–7277. DOI: https://doi.org/10.1016/j.ijhydene.2019.08.206
9. Germscheidt RL, Moreira DEB, Yoshimura RG, et al. Hydrogen Environmental Benefits Depend on the Way of Production: An Overview of the Main Processes Production and Challenges by 2050. Advanced Energy and Sustainability Research. 2021; 2(10): 2100093. DOI: https://doi.org/10.1002/aesr.202100093
10. Shao Z, Yi B. Developing Trend and Present Status of Hydrogen Energy and Fuel Cell Development. Bulletin of the Chinese Academy of Sciences. 2019; 34(4): 469–477.
11. Gonzalez-Garay A, Bui M, Freire Ordóñez D, et al. Hydrogen Production and Its Applications to Mobility. Annual Review of Chemical and Biomolecular Engineering. 2022; 13(1): 501–528. DOI: https://doi.org/10.1146/annurev-chembioeng-092220-010254
12. Roger I, Shipman MA, Symes MD. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nature Reviews Chemistry. 2017; 1(1): 0003. DOI: https://doi.org/10.1038/s41570-016-0003
13. Kamaruddin H, Jianghong Z, Yu L, et al. A review of noble metal-free high entropy alloys for water splitting applications. Journal of Materials Chemistry A. 2024; 12(17): 9933–9961. DOI: https://doi.org/10.1039/D4TA00690A
14. Kumaravel S, Karthick K, Sankar SS, et al. Current progressions in transition metal based hydroxides as bi-functional catalysts towards electrocatalytic total water splitting. Sustainable Energy & Fuels. 2021; 5(24): 6215–6268. DOI: https://doi.org/10.1039/D1SE01193F
15. Kim B, Kim T, Lee K, et al. Recent Advances in Transition Metal Phosphide Electrocatalysts for Water Splitting under Neutral pH Conditions. ChemElectroChem. 2020; 7(17): 3578–3589. DOI: https://doi.org/10.1002/celc.202000734
16. Conway BE, Tilak BV. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochimica Acta. 2002; 47(22–23): 3571–3594. DOI: https://doi.org/10.1016/S0013-4686(02)00329-8
17. Yu ZY, Duan Y, Feng XY, et al. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Advanced Materials. 2021; 33(31): 2007100. DOI: https://doi.org/10.1002/adma.202007100
18. Sun H, Yan Z, Liu F, et al. Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution. Advanced Materials. 2020; 32(3): 1806326. DOI: https://doi.org/10.1002/adma.201806326
19. Zhang XP, Chandra A, Lee YM, et al. Transition metal-mediated O–O bond formation and activation in chemistry and biology. Chemical Society Reviews. 2021; 50(8): 4804–4811. DOI: https://doi.org/10.1039/D0CS01456G
20. Wei C, Rao RR, Peng J, et al. Recommended Practices and Benchmark Activity for Hydrogen and Oxygen Electrocatalysis in Water Splitting and Fuel Cells. Advanced Materials. 2019; 31(31): 1806296. DOI: https://doi.org/10.1002/adma.201806296
21. Greeley J, Jaramillo TF, Bonde J, et al. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nature Materials. 2006; 5(11): 909–913. DOI: https://doi.org/10.1038/nmat1752
22. Greeley J, Mavrikakis M. Alloy catalysts designed from first principles. Nature Materials. 2004; 3(11): 810–815. DOI: https://doi.org/10.1038/nmat1223
23. Medford AJ, Vojvodic A, Hummelshøj JS, et al. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. Journal of Catalysis. 2015; 328: 36–42. DOI: https://doi.org/10.1016/j.jcat.2014.12.033
24. Seh ZW, Kibsgaard J, Dickens CF, et al. Combining theory and experiment in electrocatalysis: Insights into materials design. Science. 2017; 355(6321). DOI: https://doi.org/10.1126/science.aad4998
25. Montoya JH, Seitz LC, Chakthranont P, et al. Materials for solar fuels and chemicals. Nature Materials. 2017; 16(1): 70–81. DOI: https://doi.org/10.1038/nmat4778
26. Chia X, Eng AYS, Ambrosi A, et al. Electrochemistry of Nanostructured Layered Transition-Metal Dichalcogenides. Chemical Reviews. 2015; 115(21): 11941–11966. DOI: https://doi.org/10.1021/acs.chemrev.5b00287
27. Kong D, Cha JJ, Wang H, et al. First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction. Energy & Environmental Science. 2013; 6(12): 3553–3558. DOI: https://doi.org/10.1039/c3ee42413h
28. Wang Q, Lei Y, Wang Y, et al. Atomic-scale engineering of chemical-vapor-deposition-grown 2D transition metal dichalcogenides for electrocatalysis. Energy & Environmental Science. 2020; 13(6): 1593–1616. DOI: https://doi.org/10.1039/D0EE00450B
29. Wang X, Shen X, Wang Z, et al. Atomic-Scale Clarification of Structural Transition of MoS2 upon Sodium Intercalation. ACS Nano. 2014; 8(11): 11394–11400. DOI: https://doi.org/10.1021/nn505501v
30. Hong Z, Hong W, Wang B, et al. Stable 1T –2H MoS2 heterostructures for efficient electrocatalytic hydrogen evolution. Chemical Engineering Journal. 2023; 460: 141858. DOI: https://doi.org/10.1016/j.cej.2023.141858
31. Enyashin AN, Yadgarov L, Houben L, et al. New Route for Stabilization of 1T-WS2 and MoS2 Phases. The Journal of Physical Chemistry C. 2011; 115(50): 24586–24591. DOI: https://doi.org/10.1021/jp2076325
32. Huang Q, Li X, Sun M, et al. The Mechanistic Insights into the 2H-1T Phase Transition of MoS2 upon Alkali Metal Intercalation: From the Study of Dynamic Sodiation Processes of MoS2 Nanosheets. Advanced Materials Interfaces. 2017; 4(15): 1700171. DOI: https://doi.org/10.1002/admi.201700171
33. Hinnemann B, Moses PG, Bonde J, et al. Biomimetic Hydrogen Evolution: MoS2 Nanoparticles as Catalyst for Hydrogen Evolution. Journal of the American Chemical Society. 2005; 127(15): 5308–5309. DOI: https://doi.org/10.1021/ja0504690
34. Gu W, Milton RD. Natural and Engineered Electron Transfer of Nitrogenase. Chemistry. 2020; 2(2):322–346. DOI: https://doi.org/10.3390/chemistry2020021
35. Hansen JN, Prats H, Toudahl KK, et al. Is There Anything Better than Pt for HER? ACS Energy Letters. 2021; 6(4): 1175–1180. DOI: https://doi.org/10.1021/acsenergylett.1c00246
36. Jaramillo TF, Jørgensen KP, Bonde J, et al. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science. 2007; 317(5834): 100–102. DOI: https://doi.org/10.1126/science.1141483
37. Lin L, Sherrell P, Liu Y, et al. Engineered 2D Transition Metal Dichalcogenides—A Vision of Viable Hydrogen Evolution Reaction Catalysis. Advanced Energy Materials. 2020; 10(16): 1903870. DOI: https://doi.org/10.1002/aenm.201903870
38. Dong S, Wang Z. Grain Boundaries Trigger Basal Plane Catalytic Activity for the Hydrogen Evolution Reaction in Monolayer MoS2. Electrocatalysis. 2018; 9(6): 744–751. DOI: https://doi.org/10.1007/s12678-018-0485-z
39. Tsai C, Chan K, Nørskov JK, et al. Theoretical insights into the hydrogen evolution activity of layered transition metal dichalcogenides. Surface Science. 2015; 640: 133–140. DOI: https://doi.org/10.1016/j.susc.2015.01.019
40. Sekar K, Raji G, Chen S, et al. Ultrathin VS2 nanosheets vertically aligned on NiCo2S4@C3N4 hybrid for asymmetric supercapacitor and alkaline hydrogen evolution reaction. Applied Surface Science. 2020; 527: 146856. DOI: https://doi.org/10.1016/j.apsusc.2020.146856
41. Zhang Y, Shi M, Wang C, et al. Vertically aligned NiS2/CoS2/MoS2 nanosheet array as an efficient and low-cost electrocatalyst for hydrogen evolution reaction in alkaline media. Science Bulletin. 2020; 65(5): 359–366. DOI: https://doi.org/10.1016/j.scib.2019.12.003
42. Lee HJ, Lee S W, Hwang H, et al. Vertically oriented MoS2/WS2 heterostructures on reduced graphene oxide sheets as electrocatalysts for hydrogen evolution reaction. Materials Chemistry Frontiers. 2021; 5(8): 3396–3403. DOI: https://doi.org/10.1039/D1QM00051A
43. Kibsgaard J, Chen Z, Reinecke BN, et al. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nature Materials. 2012; 11(11): 963–969. DOI: https://doi.org/10.1038/nmat3439
44. Wang J, Liu J, Chao D, et al. Self-Assembly of Honeycomb-like MoS2 Nanoarchitectures Anchored into Graphene Foam for Enhanced Lithium-Ion Storage. Advanced Materials. 2014; 26(42): 7162–7169. DOI: https://doi.org/10.1002/adma.201402728
45. Wang Y, Chen B, Seo DH, et al. MoS2-coated vertical graphene nanosheet for high-performance rechargeable lithium-ion batteries and hydrogen production. NPG Asia Materials. 2016; 8(5): e268–e268. DOI: https://doi.org/10.1038/am.2016.44
46. Kong D, Wang H, Cha JJ, et al. Synthesis of MoS2 and MoSe2 Films with Vertically Aligned Layers. Nano Letters. 2013; 13(3): 1341–1347. DOI: https://doi.org/10.1021/nl400258t
47. Gong Y, Liu Z, Lupini AR, et al. Band Gap Engineering and Layer-by-Layer Mapping of Selenium-Doped Molybdenum Disulfide. Nano Letters. 2014; 14(2): 442–449. DOI: https://doi.org/10.1021/nl4032296
48. Yang Y, Fei H, Ruan G, et al. Vertically Aligned WS2 Nanosheets for Water Splitting. Advanced Functional Materials. 2015; 25(39): 6199–6204. DOI: https://doi.org/10.1002/adfm.201502479
49. Deng J, Li H, Wang S, et al. Multiscale structural and electronic control of molybdenum disulfide foam for highly efficient hydrogen production. Nature Communications. 2017; 8(1): 14430. DOI: https://doi.org/10.1038/ncomms14430
50. Choi SH, Ko YN, Lee JK, et al. 3D MoS2–Graphene Microspheres Consisting of Multiple Nanospheres with Superior Sodium Ion Storage Properties. Advanced Functional Materials. 2015; 25(12): 1780–1788. DOI: https://doi.org/10.1002/adfm.201402428
51. Zhang Z, Li W, Yuen MF, et al. Hierarchical composite structure of few-layers MoS2 nanosheets supported by vertical graphene on carbon cloth for high-performance hydrogen evolution reaction. Nano Energy. 2015; 18: 196–204. DOI: https://doi.org/10.1016/j.nanoen.2015.10.014
52. Dong H, Liu C, Ye H, et al. Three-dimensional Nitrogen-Doped Graphene Supported Molybdenum Disulfide Nanoparticles as an Advanced Catalyst for Hydrogen Evolution Reaction. Scientific Reports. 2015; 5(1): 17542. DOI: https://doi.org/10.1038/srep17542
53. Zhao Y, Kuai L, Liu Y, et al. Well-Constructed Single-Layer Molybdenum Disulfide Nanorose Cross-Linked by Three Dimensional-Reduced Graphene Oxide Network for Superior Water Splitting and Lithium Storage Property. Scientific Reports. 2015; 5(1): 8722. DOI: https://doi.org/10.1038/srep08722
54. Zhou W, Zhou K, Hou D, et al. Three-Dimensional Hierarchical Frameworks Based on MoS2 Nanosheets Self-Assembled on Graphene Oxide for Efficient Electrocatalytic Hydrogen Evolution. ACS Applied Materials & Interfaces. 2014; 6(23): 21534–21540. DOI: https://doi.org/10.1021/am506545g
55. Zhao Y, Xie X, Zhang J, et al. MoS2 Nanosheets Supported on 3D Graphene Aerogel as a Highly Efficient Catalyst for Hydrogen Evolution. Chemistry—A European Journal. 2015; 21(45): 15908–15913. DOI: https://doi.org/10.1002/chem.201501964
56. Tan Y, Liu P, Chen L, et al. Monolayer MoS2 Films Supported by 3D Nanoporous Metals for High-Efficiency Electrocatalytic Hydrogen Production. Advanced Materials. 2014; 26(47): 8023–8028. DOI: https://doi.org/10.1002/adma.201403808
57. Chang YH, Lin CT, Chen TY, et al. Highly Efficient Electrocatalytic Hydrogen Production by MoSx Grown on Graphene-Protected 3D Ni Foams. Advanced Materials. 2013; 25(5): 756–760. DOI: https://doi.org/10.1002/adma.201202920
58. Novoselov KS, Geim AK, Morozov SV, et al. Electric Field Effect in Atomically Thin Carbon Films. Science. 2004; 306(5696): 666. DOI: https://doi.org/10.1126/science.1102896
59. Novoselov KS, Fal’ko VI, Colombo L, et al. A roadmap for graphene. Nature. 2012; 490(7419): 192–200. DOI: https://doi.org/10.1038/nature11458
60. Geim AK, Novoselov KS. The rise of graphene. Nature Materials. 2007; 6(3): 183–191. DOI: https://doi.org/10.1038/nmat1849
61. Castro Neto AH, Guinea F, Peres NMR, et al. The electronic properties of graphene. Reviews of Modern Physics. 2009; 81(1): 109–162. DOI: https://doi.org/10.1103/RevModPhys.81.109
62. Mao S, Yu K, Chang J, et al. Direct Growth of Vertically-oriented Graphene for Field-Effect Transistor Biosensor. Scientific Reports. 2013; 3(1): 1696. DOI: https://doi.org/10.1038/srep01696
63. Bo Z, Yu K, Lu G, et al. Understanding growth of carbon nanowalls at atmospheric pressure using normal glow discharge plasma-enhanced chemical vapor deposition. Carbon. 2011; 49(6): 1849–1858. DOI: https://doi.org/10.1016/j.carbon.2011.01.007
64. Lee JS, Kim SI, Yoon JC, et al. Chemical Vapor Deposition of Mesoporous Graphene Nanoballs for Supercapacitor. ACS Nano. 2013; 7(7): 6047–6055. DOI: https://doi.org/10.1021/nn401850z
65. Sohn K, Joo Na Y, Chang H, et al. Oil absorbing graphene capsules by capillary molding. Chemical Communications. 2012; 48(48): 5968–5970. DOI: https://doi.org/10.1039/c2cc32049e
66. Huang X, Qian K, Yang J, et al. Functional Nanoporous Graphene Foams with Controlled Pore Sizes. Advanced Materials. 2012; 24(32): 4419–4423. DOI: https://doi.org/10.1002/adma.201201680
67. Yao HB, Ge J, Wang CF, et al. A Flexible and Highly Pressure-Sensitive Graphene–Polyurethane Sponge Based on Fractured Microstructure Design. Advanced Materials. 2013; 25(46): 6692–6698. DOI: https://doi.org/10.1002/adma.201303041
68. Zhu C, Han TYJ, Duoss EB, et al. Highly compressible 3D periodic graphene aerogel microlattices. Nature Communications. 2015; 6(1): 6962. DOI: https://doi.org/10.1038/ncomms7962
69. Zhang Q, Zhang F, Medarametla SP, et al. 3D Printing of Graphene Aerogels. Small. 2016; 12(13): 1702–1708. DOI: https://doi.org/10.1002/smll.201503524
70. Huang K, Yang J, Dong S, et al. Anisotropy of graphene scaffolds assembled by three-dimensional printing. Carbon. 2018; 130: 1–10. DOI: https://doi.org/10.1016/j.carbon.2017.12.120
71. Bai H, Li C, Wang X, et al. On the Gelation of Graphene Oxide. The Journal of Physical Chemistry C. 2011; 115(13): 5545–5551. DOI: https://doi.org/10.1021/jp1120299
72. Jiang X, Ma Y, Li J, et al. Self-Assembly of Reduced Graphene Oxide into Three-Dimensional Architecture by Divalent Ion Linkage. The Journal of Physical Chemistry C. 2010; 114(51): 22462–22465. DOI: https://doi.org/10.1021/jp108081g
73. Cong HP, Ren XC, Wang P, et al. Macroscopic Multifunctional Graphene-Based Hydrogels and Aerogels by a Metal Ion Induced Self-Assembly Process. ACS Nano. 2012; 6(3): 2693–2703. DOI: https://doi.org/10.1021/nn300082k
74. Su Y, Zhang Y, Zhuang X, et al. Low-temperature synthesis of nitrogen/sulfur co-doped three-dimensional graphene frameworks as efficient metal-free electrocatalyst for oxygen reduction reaction. Carbon. 2013; 62: 296–301. DOI: https://doi.org/10.1016/j.carbon.2013.05.067
75. Tang Z, Shen S, Zhuang J, et al. Noble-Metal-Promoted Three-Dimensional Macroassembly of Single-Layered Graphene Oxide. Angewandte Chemie International Edition. 2010; 49(27): 4603–4607. DOI: https://doi.org/10.1002/anie.201000270
76. Wu Y, Zhu J, Huang L. A review of three-dimensional graphene-based materials: Synthesis and applications to energy conversion/storage and environment. Carbon. 2019; 143: 610–640. DOI: https://doi.org/10.1016/j.carbon.2018.11.053
77. Mao J, Iocozzia J, Huang J, et al. Graphene aerogels for efficient energy storage and conversion. Energy & Environmental Science. 2018; 11(4): 772–799. DOI: https://doi.org/10.1039/C7EE03031B
78. Qu Y, Medina H, Wang SW, et al. Wafer Scale Phase-Engineered 1T- and 2H-MoSe2/Mo Core–Shell 3D-Hierarchical Nanostructures toward Efficient Electrocatalytic Hydrogen Evolution Reaction. Advanced Materials. 2016; 28(44): 9831–9838. DOI: https://doi.org/10.1002/adma.201602697
79. Chia X, Pumera M. Characteristics and performance of two-dimensional materials for electrocatalysis. Nature Catalysis. 2018; 1(12): 909–921. DOI: https://doi.org/10.1038/s41929-018-0181-7
80. Jin H, Guo C, Liu X, et al. Emerging Two-Dimensional Nanomaterials for Electrocatalysis. Chemical Reviews. 2018; 118(13): 6337–6408. DOI: https://doi.org/10.1021/acs.chemrev.7b00689
81. Kertesz M, Hoffmann R. Octahedral vs. trigonal-prismatic coordination and clustering in transition-metal dichalcogenides. Journal of the American Chemical Society. 1984; 106(12): 3453–3460. DOI: https://doi.org/10.1021/ja00324a012
82. Voiry D, Yamaguchi H, Li J, et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nature Materials. 2013; 12(9): 850–855. DOI: https://doi.org/10.1038/nmat3700
83. Voiry D, Salehi M, Silva R, et al. Conducting MoS2 Nanosheets as Catalysts for Hydrogen Evolution Reaction. Nano Letters. 2013; 13(12): 6222–6227. DOI: https://doi.org/10.1021/nl403661s
84. Zhu J, Wang Z, Yu H, et al. Argon Plasma Induced Phase Transition in Monolayer MoS2. Journal of the American Chemical Society. 2017; 139(30): 10216–10219. DOI: https://doi.org/10.1021/jacs.7b05765
85. Cheng H, Yang N, Liu G, et al. Ligand-Exchange-Induced Amorphization of Pd Nanomaterials for Highly Efficient Electrocatalytic Hydrogen Evolution Reaction. Advanced Materials. 2020; 32(11): 1902964. DOI: https://doi.org/10.1002/adma.201902964
86. Merki D, Fierro S, Vrubel H, et al. Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water. Chemical Science. 2011; 2(7): 1262–1267. DOI: https://doi.org/10.1039/C1SC00117E
87. Vrubel H, Merki D, Hu X. Hydrogen evolution catalyzed by MoS3 and MoS2 particles. Energy & Environmental Science. 2012; 5(3): 6136–6144. DOI: https://doi.org/10.1039/c2ee02835b
88. Ting LRL, Deng Y, Ma L, et al. Catalytic Activities of Sulfur Atoms in Amorphous Molybdenum Sulfide for the Electrochemical Hydrogen Evolution Reaction. ACS Catalysis. 2016; 6(2): 861–867. DOI: https://doi.org/10.1021/acscatal.5b02369
89. Deng Y, Ting LRL, Neo PHL, et al. Operando Raman Spectroscopy of Amorphous Molybdenum Sulfide (MoSx) during the Electrochemical Hydrogen Evolution Reaction: Identification of Sulfur Atoms as Catalytically Active Sites for H+ Reduction. ACS Catalysis. 2016; 6(11): 7790–7798. DOI: https://doi.org/10.1021/acscatal.6b01848
90. Dinda D, Ahmed ME, Mandal S, et al. Amorphous molybdenum sulfide quantum dots: an efficient hydrogen evolution electrocatalyst in neutral medium. Journal of Materials Chemistry A. 2016; 4(40): 15486–15493. DOI: https://doi.org/10.1039/C6TA06101J
91. Benck JD, Chen Z, Kuritzky LY, et al. Amorphous Molybdenum Sulfide Catalysts for Electrochemical Hydrogen Production: Insights into the Origin of their Catalytic Activity. ACS Catalysis. 2012; 2(9): 1916–1923. DOI: https://doi.org/10.1021/cs300451q
92. Cao PF, Hu Y, Zhang YW, et al. Radiation Induced Synthesis of Amorphous Molybdenum Sulfide/Reduced Graphene Oxide Nanocomposites for Efficient Hydrogen Evolution Reaction. Acta Physico-Chimica Sinica. 2017; 33(12): 2542–2549.
93. Wang T, Liu L, Zhu Z, et al. Enhanced electrocatalytic activity for hydrogen evolution reaction from self-assembled monodispersed molybdenum sulfide nanoparticles on an Au electrode. Energy & Environmental Science. 2013; 6(2): 625–633. DOI: https://doi.org/10.1039/C2EE23513G
94. Li DJ, Maiti UN, Lim J, et al. Molybdenum Sulfide/N-Doped CNT Forest Hybrid Catalysts for High-Performance Hydrogen Evolution Reaction. Nano Letters. 2014; 14(3): 1228–1233. DOI: https://doi.org/10.1021/nl404108a
95. Tang YJ, Wang Y, Wang XL, et al. Molybdenum Disulfide/Nitrogen-Doped Reduced Graphene Oxide Nanocomposite with Enlarged Interlayer Spacing for Electrocatalytic Hydrogen Evolution. Advanced Energy Materials. 2016; 6(12): 1600116. DOI: https://doi.org/10.1002/aenm.201600116
96. Gao MR, Chan MKY, Sun Y. Edge-terminated molybdenum disulfide with a 9.4-Å interlayer spacing for electrochemical hydrogen production. Nature Communications. 2015; 6(1): 7493. DOI: https://doi.org/10.1038/ncomms8493
97. Xu Y, Wang L, Liu X, et al. Monolayer MoS2 with S vacancies from interlayer spacing expanded counterparts for highly efficient electrochemical hydrogen production. Journal of Materials Chemistry A. 2016; 4(42): 16524–16530. DOI: https://doi.org/10.1039/C6TA06534A
98. Zhu H, Zhang J, Yanzhang R, et al. When Cubic Cobalt Sulfide Meets Layered Molybdenum Disulfide: A Core–Shell System Toward Synergetic Electrocatalytic Water Splitting. Advanced Materials. 2015; 27(32): 4752–4759. DOI: https://doi.org/10.1002/adma.201501969
99. Xu X, Zhong W, Zhang L, et al. Synergistic effect of MoS2 and Ni9S8 nanosheets as an efficient electrocatalyst for hydrogen evolution reaction. Journal of Colloid and Interface Science. 2019; 556: 24–32. DOI: https://doi.org/10.1016/j.jcis.2019.08.041
100. Du C, Liang D, Shang M, et al. In Situ Engineering MoS2 NDs/VS2 Lamellar Heterostructure for Enhanced Electrocatalytic Hydrogen Evolution. ACS Sustainable Chemistry & Engineering. 2018; 6(11): 15471–15479. DOI: https://doi.org/10.1021/acssuschemeng.8b03929
101. Hu J, Zhang C, Zhang Y, et al. Interface Modulation of MoS2/Metal Oxide Heterostructures for Efficient Hydrogen Evolution Electrocatalysis. Small. 2020; 16(28): 2002212. DOI: https://doi.org/10.1002/smll.202002212
102. Wu Q, Luo Y, Xie R, et al. Space-Confined One-Step Growth of 2D MoO2/MoS2 Vertical Heterostructures for Superior Hydrogen Evolution in Alkaline Electrolytes. Small. 2022; 18(32): 2201051. DOI: https://doi.org/10.1002/smll.202201051
103. Duraisamy S, Ganguly A, Sharma PK, et al. One-Step Hydrothermal Synthesis of Phase-Engineered MoS2/MoO3 Electrocatalysts for Hydrogen Evolution Reaction. ACS Applied Nano Materials. 2021; 4(3): 2642–2656. DOI: https://doi.org/10.1021/acsanm.0c03274
104. Luo Y, Tang L, Khan U, et al. Morphology and surface chemistry engineering toward pH-universal catalysts for hydrogen evolution at high current density. Nature Communications. 2019; 10(1): 269. DOI: https://doi.org/10.1038/s41467-018-07792-9
105. Ren J, Zong H, Sun Y, et al. 2D organ-like molybdenum carbide (MXene) coupled with MoS2 nanoflowers enhances the catalytic activity in the hydrogen evolution reaction. CrystEngComm. 2020; 22(8): 1395–1403. DOI: https://doi.org/10.1039/C9CE01777A
106. Kim M, Anjum MAR, Lee M, et al. Activating MoS2 Basal Plane with Ni2P Nanoparticles for Pt-Like Hydrogen Evolution Reaction in Acidic Media. Advanced Functional Materials. 2019; 29(10): 1809151. DOI: https://doi.org/10.1002/adfm.201809151
107. Song L, Wang X, Wen F, et al. Hydrogen evolution reaction performance of the molybdenum disulfide/nickel–phosphorus composites in alkaline solution. International Journal of Hydrogen Energy. 2016; 41(42): 18942–18952. DOI: https://doi.org/10.1016/j.ijhydene.2016.09.014
108. Yang F, Kang N, Yan J, et al. Hydrogen Evolution Reaction Property of Molybdenum Disulfide/Nickel Phosphide Hybrids in Alkaline Solution. Metals. 2018; 8(5): 359. DOI: https://doi.org/10.3390/met8050359
109. Hu J, Zhang C, Jiang L, et al. Nanohybridization of MoS2 with Layered Double Hydroxides Efficiently Synergizes the Hydrogen Evolution in Alkaline Media. Joule. 2017; 1(2): 383–393. DOI: https://doi.org/10.1016/j.joule.2017.07.011
110. Zhang B, Liu J, Wang J, et al. Interface engineering: The Ni(OH)2/MoS2 heterostructure for highly efficient alkaline hydrogen evolution. Nano Energy. 2017; 37: 74–80. DOI: https://doi.org/10.1016/j.nanoen.2017.05.011
111. Luo Y, Li X, Cai X, et al. Two-Dimensional MoS2 Confined Co(OH)2 Electrocatalysts for Hydrogen Evolution in Alkaline Electrolytes. ACS Nano. 2018; 12(5): 4565–4573. DOI: https://doi.org/10.1021/acsnano.8b00942
112. Kitchin JR, Nørskov JK, Barteau MA, et al. Trends in the chemical properties of early transition metal carbide surfaces: A density functional study. Catalysis Today. 2005; 105(1): 66–73. DOI: https://doi.org/10.1016/j.cattod.2005.04.008
113. Liu C, Wen Y, Lin L, et al. Facile in-situ formation of high efficiency nanocarbon supported tungsten carbide nanocatalysts for hydrogen evolution reaction. International Journal of Hydrogen Energy. 2018; 43(33): 15650–15658. DOI: https://doi.org/10.1016/j.ijhydene.2018.06.087
114. Ham DJ, Lee JS. Transition Metal Carbides and Nitrides as Electrode Materials for Low Temperature Fuel Cells. Energies. 2009; 2(4) :873–899. DOI: https://doi.org/10.3390/en20400873
115. Velpandian M, Ragunathan A, Ummethala G, et al. Low-Potential Overall Water Splitting Induced by Engineered CoTe2–WTe2 Heterointerfaces. ACS Applied Energy Materials. 2023; 6(11): 5968–5978. DOI: https://doi.org/10.1021/acsaem.3c00412
116. Zheng Y, Jiao Y, Jaroniec M, et al. Advancing the Electrochemistry of the Hydrogen-Evolution Reaction through Combining Experiment and Theory. Angewandte Chemie International Edition. 2015; 54(1): 52–65. DOI: https://doi.org/10.1002/anie.201407031
117. Wang X, Zhang Y, Si H, et al. Single-Atom Vacancy Defect to Trigger High-Efficiency Hydrogen Evolution of MoS2. Journal of the American Chemical Society. 2020; 142(9): 4298–4308. DOI: https://doi.org/10.1021/jacs.9b12113
118. Sun X, Dai J, Guo Y, et al. Semimetallic molybdenum disulfide ultrathin nanosheets as an efficient electrocatalyst for hydrogen evolution. Nanoscale. 2014; 6(14): 8359–8367. DOI: https://doi.org/10.1039/C4NR01894J
119. Qi K, Yu S, Wang Q, et al. Decoration of the inert basal plane of defect-rich MoS2 with Pd atoms for achieving Pt-similar HER activity. Journal of Materials Chemistry A. 2016; 4(11): 4025–4031. DOI: https://doi.org/10.1039/C5TA10337A
120. Wang H, Tsai C, Kong D, et al. Transition-metal doped edge sites in vertically aligned MoS2 catalysts for enhanced hydrogen evolution. Nano Research. 2015; 8(2): 566–575. DOI: https://doi.org/10.1007/s12274-014-0677-7
121. Deng J, Li H, Xiao J, et al. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy & Environmental Science. 2015; 8(5): 1594–1601. DOI: https://doi.org/10.1039/C5EE00751H
122. Sun C, Zhang J, Ma J, et al. N-doped WS2 nanosheets: a high-performance electrocatalyst for the hydrogen evolution reaction. Journal of Materials Chemistry A. 2016; 4(29): 11234–11238. DOI: https://doi.org/10.1039/C6TA04082A
123. Shifa TA, Wang F, Liu K, et al. Efficient Catalysis of Hydrogen Evolution Reaction from WS2(1−x)P2x Nanoribbons. Small. 2017; 13(16): 1603706. DOI: https://doi.org/10.1002/smll.201603706
124. Xie J, Zhang J, Li S, et al. Correction to Controllable Disorder Engineering in Oxygen-Incorporated MoS2 Ultrathin Nanosheets for Efficient Hydrogen Evolution. Journal of the American Chemical Society. 2014; 136(4): 1680–1680. DOI: https://doi.org/10.1021/ja4129636
125. Liu P, Zhu J, Zhang J, et al. P Dopants Triggered New Basal Plane Active Sites and Enlarged Interlayer Spacing in MoS2 Nanosheets toward Electrocatalytic Hydrogen Evolution. ACS Energy Letters. 2017; 2(4): 745–752. DOI: https://doi.org/10.1021/acsenergylett.7b00111
126. Zhang G, Zheng X, Xu Q, et al. Carbon nanotube-induced phase and stability engineering: a strained cobalt-doped WSe2/MWNT heterostructure for enhanced hydrogen evolution reaction. Journal of Materials Chemistry A. 2018; 6(11): 4793–4800. DOI: https://doi.org/10.1039/C8TA00163D
127. Zhang W, Liu X, Liu T, et al. In Situ Investigation on Doping Effect in Co-Doped Tungsten Diselenide Nanosheets for Hydrogen Evolution Reaction. The Journal of Physical Chemistry C. 2021; 125(11): 6229–6236. DOI: https://doi.org/10.1021/acs.jpcc.0c11552
128. Merki D, Vrubel H, Rovelli L, et al. Fe, Co, and Ni ions promote the catalytic activity of amorphous molybdenum sulfide films for hydrogen evolution. Chemical Science. 2012; 3(8); 2515–2525. DOI: https://doi.org/10.1039/c2sc20539d
129. Luo Y, Zhang Z, Chhowalla M, et al. Recent Advances in Design of Electrocatalysts for High-Current-Density Water Splitting. Advanced Materials. 2022; 34(16): 2108133. DOI: https://doi.org/10.1002/adma.202108133
130. Lu AY, Yang X, Tseng CC, et al. High-Sulfur-Vacancy Amorphous Molybdenum Sulfide as a High Current Electrocatalyst in Hydrogen Evolution. Small. 2016; 12(40): 5530–5537. DOI: https://doi.org/10.1002/smll.201602107
131. Azcatl A, Qin X, Prakash A, et al. Covalent Nitrogen Doping and Compressive Strain in MoS2 by Remote N2 Plasma Exposure. Nano Letters. 2016; 16(9): 5437–5443. DOI: https://doi.org/10.1021/acs.nanolett.6b01853
132. Zeng K, Zhang D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Progress in Energy and Combustion Science. 2010; 36(3): 307–326. DOI: https://doi.org/10.1016/j.pecs.2009.11.002
133. Smith RDL, Prévot MS, Fagan RD, et al. Photochemical Route for Accessing Amorphous Metal Oxide Materials for Water Oxidation Catalysis. Science. 2013; 340(6128): 60–63. DOI: https://doi.org/10.1126/science.1233638




.jpg)
.jpg)

