Numerical investigation of laminar hydrogen combustion across multiple flame configurations under pressure and strain effects
DOI:
https://doi.org/10.18686/cest525Keywords:
combustion; hydrogen; laminar; flames; CanteraAbstract
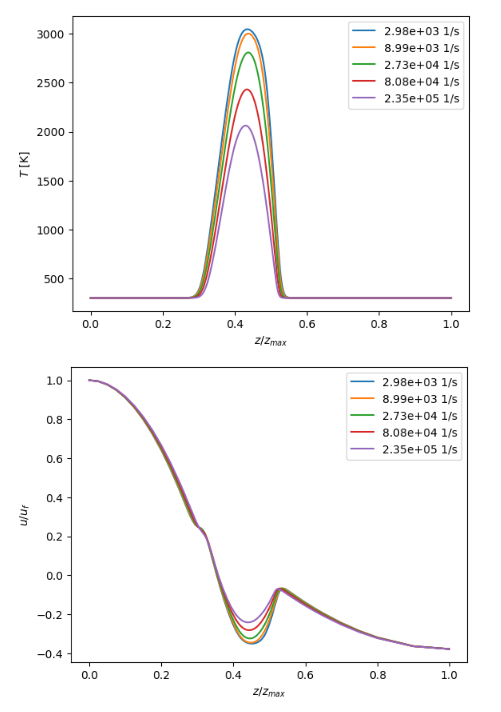
This research quantitatively examines laminar hydrogen combustion under diverse pressure and strain conditions, employing comprehensive chemical kinetics in Cantera 3.0 with the H2–O2 and GRI-Mech 3.0 mechanisms. To learn more about flame speed, structure, and extinction behavior, we looked at four main flame configurations: freely propagating premixed, counterflow diffusion, premixed counterflow, and stagnation-point flames. The laminar flame speed of pure hydrogen was 310 cm/s, which means it burned very quickly because it spread out quickly. Hydrogen stayed stable up to a strain rate of 6.0 × 105 s–1 before it went out. The peak flame temperature dropped from 3600 K to 3000 K when the pressure rose from 1 bar to 100 bar. This shows that higher pressure makes things more stable but less heat is released. The innovation of this study resides in the integration of all principal flame configurations into a cohesive modeling framework, demonstrating that strain rate exerts a more significant impact on flame collapse than pressure. These results give us a baseline for designing efficient turbines, rocket combustors, and industrial heating systems that run on hydrogen.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2026 Author

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
1. Shchepakina EA, Zubrilin IA, Kuznetsov AY, et al. Physical and chemical features of hydrogen combustion and their influence on the characteristics of gas turbine combustion chambers. Applied Sciences. 2023; 13(6): 3754. doi: 10.3390/app13063754 DOI: https://doi.org/10.3390/app13063754
2. Giacomazzi E, Troiani G, Di Nardo A, et al. Hydrogen combustion: Features and barriers to its exploitation in the energy transition. Energies. 2023; 16(20): 7174. doi: 10.3390/en16207174 DOI: https://doi.org/10.3390/en16207174
3. Jeon J, Kim SJ. Recent progress in hydrogen flammability prediction for the safe energy systems. Energies. 2020; 13(23): 6263. doi: 10.3390/en13236263 DOI: https://doi.org/10.3390/en13236263
4. Calabrese M, Portarapillo M, Di Nardo A, et al. Hydrogen safety challenges: a comprehensive review on production, storage, transport, utilization, and CFD-based consequence and risk assessment. Energies. 2024; 17(6): 1350. doi: 10.3390/en17061350 DOI: https://doi.org/10.3390/en17061350
5. Yip HL, Srna A, Yuen ACY, et al. A review of hydrogen direct injection for internal combustion engines: towards carbon-free combustion. Applied Sciences. 2019; 9(22): 4842. doi: 10.3390/app9224842 DOI: https://doi.org/10.3390/app9224842
6. Lan H, Wang G, Zhao K, et al. Review on the hydrogen dispersion and the burning behavior of fuel cell electric vehicles. Energies. 2022; 15(19): 7295. doi: 10.3390/en15197295 DOI: https://doi.org/10.3390/en15197295
7. Mehdi G, De Giorgi MG, Bonuso S, et al. Comparative analysis of flame propagation and flammability limits of CH4/H2/air mixture with or without nanosecond plasma discharges. Aerospace. 2023; 10(3): 224. doi: 10.3390/aerospace10030224 DOI: https://doi.org/10.3390/aerospace10030224
8. Ricci F, Zembi J, Avana M, et al. Analysis of hydrogen combustion in a spark ignition research engine with a barrier discharge igniter. Energies. 2024; 17(7): 1739. doi: 10.3390/en17071739 DOI: https://doi.org/10.3390/en17071739
9. Habib MA, Abdulrahman GAQ, Alquaity ABS, et al. Hydrogen combustion, production, and applications: A review. Alexandria Engineering Journal. 2024; 100: 182-207. doi: 10.1016/j.aej.2024.05.030 DOI: https://doi.org/10.1016/j.aej.2024.05.030
10. Han W, Dai P, Gou X, et al. A review of laminar flame speeds of hydrogen and syngas measured from propagating spherical flames. Applications in Energy and Combustion Science. 2020; 1: 100008. doi: 10.1016/j.jaecs.2020.100008 DOI: https://doi.org/10.1016/j.jaecs.2020.100008
11. Sánchez AL, Williams FA. Recent advances in understanding of flammability characteristics of hydrogen. Progress in Energy and Combustion Science. 2014; 41: 1-55. doi: 10.1016/j.pecs.2013.10.002 DOI: https://doi.org/10.1016/j.pecs.2013.10.002
12. Ravi S, Petersen EL. Laminar flame speed correlations for pure-hydrogen and high-hydrogen content syngas blends with various diluents. International Journal of Hydrogen Energy. 2012; 37(24): 19177-19189. doi: 10.1016/j.ijhydene.2012.09.086 DOI: https://doi.org/10.1016/j.ijhydene.2012.09.086
13. Kuznetsov M, Redlinger R, Breitung W, et al. Laminar burning velocities of hydrogen-oxygen-steam mixtures at elevated temperatures and pressures. Proceedings of the Combustion Institute. 2011; 33(1): 895-903. doi: 10.1016/j.proci.2010.06.050 DOI: https://doi.org/10.1016/j.proci.2010.06.050
14. Burke MP, Chaos M, Dryer FL, et al. Negative pressure dependence of mass burning rates of H2/CO/O2/diluent flames at low flame temperatures. Combustion and Flame. 2010; 157(4): 618-631. doi: 10.1016/j.combustflame.2009.08.009 DOI: https://doi.org/10.1016/j.combustflame.2009.08.009
15. Pfahl UJ, Ross MC, Shepherd JE, et al. Flammability limits, ignition energy, and flame speeds in H2–CH4–NH3–N2O–O2–N2 mixtures. Combustion and Flame. 2000; 123(1-2): 140-158. doi: 10.1016/S0010-2180(00)00152-8 DOI: https://doi.org/10.1016/S0010-2180(00)00152-8
16. Butler MS, Moran CW, Sunderland PB, et al. Limits for hydrogen leaks that can support stable flames. International Journal of Hydrogen Energy. 2009; 34(12): 5174-5182. doi: 10.1016/j.ijhydene.2009.04.012 DOI: https://doi.org/10.1016/j.ijhydene.2009.04.012
17. Song W, Dave H, Im HG, et al. Local flame displacement speeds of hydrogen-air premixed flames in moderate to intense turbulence. Combustion and Flame. 2022; 236: 111812. doi: 10.1016/j.combustflame.2021.111812 DOI: https://doi.org/10.1016/j.combustflame.2021.111812
18. Yilmaz H, Schröder L, Hillenbrand T, et al. Effects of hydrogen addition on combustion and flame propagation characteristics of laser ignited methane/air mixtures. International Journal of Hydrogen Energy. 2023; 48(45): 17324-17338. doi: 10.1016/j.ijhydene.2023.01.224 DOI: https://doi.org/10.1016/j.ijhydene.2023.01.224
19. El-Ghafour SAA, El-Dein AHE, Aref AAR. Combustion characteristics of natural gas–hydrogen hybrid fuel turbulent diffusion flame. International Journal of Hydrogen Energy. 2010; 35(6): 2556-2565. doi: 10.1016/j.ijhydene.2009.12.049 DOI: https://doi.org/10.1016/j.ijhydene.2009.12.049
20. Amez I, Paredes R, León D, et al. New Experimental Approaches for the Determination of Flammability Limits in Methane–Hydrogen Mixtures with CO2 Inertization Using the Spark Test Apparatus. Fire. 2024; 7(11): 403. doi: 10.3390/fire7110403 DOI: https://doi.org/10.3390/fire7110403
21. Nemitallah MA, Habib MA, Abdelhafez A. Hydrogen for Clean Energy Production: Combustion Fundamentals and Applications. Springer Nature; 2024. doi: 10.1007/978-981-97-7925-3 DOI: https://doi.org/10.1007/978-981-97-7925-3
22. Kumar S, Agarwal AK, Khandelwal B, Singh P (editors). Introduction to Ammonia and Hydrogen for Green Energy Transition. In: Ammonia and Hydrogen for Green Energy Transition. Energy, Environment, and Sustainability. Springer; 2024. doi: 10.1007/978-981-97-0507-8_1 DOI: https://doi.org/10.1007/978-981-97-0507-8
23. Das LM. Hydrogen Engines: Design, Performance Evaluation, Combustion Analysis, and Exhaust Emissions. John Wiley & Sons; 2024. DOI: https://doi.org/10.1002/9781394245710
24. Gelfand BE, Silnikov MV, Medvedev SP, et al. Thermo-Gas Dynamics of Hydrogen Combustion and Explosion. Springer; 2012. DOI: https://doi.org/10.1007/978-3-642-25352-2
25. Tingas EA (editor). Hydrogen for Future Thermal Engines. Springer; 2023. DOI: https://doi.org/10.1007/978-3-031-28412-0
26. Alverà M. The Hydrogen Revolution: A Blueprint for the Future of Clean Energy. Hodder & Stoughton; 2022.
27. Glassman I, Yetter RA, Glumac NG. Combustion, 5th ed. Elsevier; 2014.
28. Turns SR. An Introduction to Combustion: Concepts and Applications, 4th ed. McGraw-Hill; 2021.
29. Goodwin DG, Moffat HK, Schoegl I, et al. Cantera: An object-oriented software toolkit for chemical kinetics, thermodynamics, and transport processes. Available online: https://zenodo.org/records/14455267 (accessed on 1 December 2025).




.jpg)
.jpg)

