High-performance proton exchange membrane employing water-insoluble hybrid formed by chemically bonding phosphotungstic acid with polydopamine
DOI:
https://doi.org/10.18686/cest.v2i2.138Keywords:
proton exchange membrane for fuel cell; sulfonated poly(ether ether ketone); phosphotungstic acid; polydopamine; hydrothermalAbstract
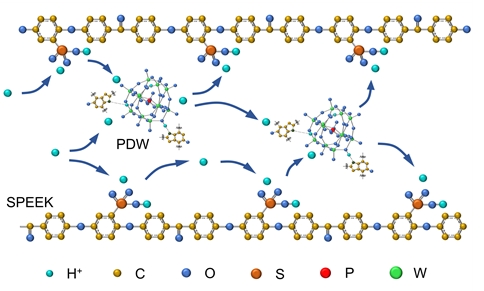
Heteropolyacids can retain water in a proton exchange membrane to increase proton conductivity at high temperatures and low humidity; however, their high solubility in water leads to leaching, which limits their further application. Herein, we used phosphotungstic acid (HPW) and polydopamine (PDA) particles to prepare a water-insoluble PDA/HPW hybrid (PDW) via hydrothermal reaction. The amino groups of PDA in PDW chemically bonded to HPW and acted as an anchor for HPW. The proton conductivity of the sulfonated poly(ether ether ketone) (SPEEK) composite membrane containing 15wt% PDW (SPEEK/PDW-15) in liquid water was 0.052 S⸱cm–1 at 25 ℃, which was 63% higher than that of the SPEEK control membrane (0.032 S⸱cm–1). The SPEEK/PDW-15 composite membrane also showed stable proton conductivity during 80 days of testing while immersed in water.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Zhongrui Lu, Xiancan Yuan, Xiaoyang Jia, Jun Lin, Shaojian He

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
1. Yang W, Zhang E, Zhao J, et al. Dawn of clean energy: Enhanced heat transfer, radiative cooling, and firecracker-style controlled nuclear fusion power generation system. Clean Energy Science and Technology 2023; 1(1): 61. doi: 10.18686/cest.v1i1.61 DOI: https://doi.org/10.18686/cest.v1i1.61
2. Lu G, Wang Z, Bhatti UH, et al. Recent progress in carbon dioxide capture technologies: A review. Clean Energy Science and Technology 2023; 1(1): 32. doi: 10.18686/cest.v1i1.32 DOI: https://doi.org/10.18686/cest.v1i1.32
3. Zheng J, Chen X, Ma J. Advances in solid adsorbent materials for direct air capture of CO2. Clean Energy Science and Technology 2023; 1(2): 95. doi: 10.18686/cest.v1i2.95 DOI: https://doi.org/10.18686/cest.v1i2.95
4. Chen J, Zhang W, Yang W, et al. Separation of benzene and toluene associated with vapochromic behaviors by hybrid 4 arene-based co-crystals. Nature Communications 2024; 15(1): 1260. doi: 10.1038/s41467-024-45592-6 DOI: https://doi.org/10.1038/s41467-024-45592-6
5. Yan M, Wang Y, Chen J, et al. Potential of nonporous adaptive crystals for hydrocarbon separation. Chemical Society Reviews 2023; 52(17): 6075-6119. doi: 10.1039/d2cs00856d DOI: https://doi.org/10.1039/D2CS00856D
6. Zhang R, Zhou J. Ultrafast-adsorption-kinetics molecular sieving of propylene from propane. Clean Energy Science and Technology 2024; 2(2): 126. doi: 10.18686/cest.v2i2.126 DOI: https://doi.org/10.18686/cest.v2i2.126
7. Harun NAM, Shaari N, Nik Zaiman NFH. A review of alternative polymer electrolyte membrane for fuel cell application based on sulfonated poly(ether ether ketone). International Journal of Energy Research 2021; 45(14): 19671-19708. doi: 10.1002/er.7048 DOI: https://doi.org/10.1002/er.7048
8. Nimir W, AlOthman A, Tawalbeh M, et al. Approaches towards the development of heteropolyacid-based high temperature membranes for PEM fuel cells. International Journal of Hydrogen Energy 2023; 48(17): 6638-6656. doi: 10.1016/j.ijhydene.2021.11.174 DOI: https://doi.org/10.1016/j.ijhydene.2021.11.174
9. Geiling J, Steinberger M, Ortner F, et al. Combined dynamic operation of PEM fuel cell and continuous dehydrogenation of perhydro-dibenzyltoluene. International Journal of Hydrogen Energy 2021; 46(72): 35662-35677. doi: 10.1016/j.ijhydene.2021.08.034 DOI: https://doi.org/10.1016/j.ijhydene.2021.08.034
10. Kim AR, Poudel MB, Chu JY, et al. Advanced performance and ultra-high, long-term durability of acid-base blended membranes over 900 hours containing sulfonated PEEK and quaternized poly(arylene ether sulfone) in H2/O2 fuel cells. Composites Part B-Engineering 2023; 254: 110558. doi: 10.1016/j.compositesb.2023.110558 DOI: https://doi.org/10.1016/j.compositesb.2023.110558
11. Rauf M, Wang JW, Zhang P, et al. Non-precious nanostructured materials by electrospinning and their applications for oxygen reduction in polymer electrolyte membrane fuel cells. Journal of Power Sources 2018; 408: 17-27. doi: 10.1016/j.jpowsour.2018.10.074 DOI: https://doi.org/10.1016/j.jpowsour.2018.10.074
12. Elwan HA, Mamlouk M, Scott K. A review of proton exchange membranes based on protic ionic liquid/polymer blends for polymer electrolyte membrane fuel cells. Journal of Power Sources 2021; 484: 229197. doi: 10.1016/j.jpowsour.2020.229197 DOI: https://doi.org/10.1016/j.jpowsour.2020.229197
13. Kim AR, Vinothkannan M, Song MH, et al. Amine functionalized carbon nanotube (ACNT) filled in sulfonated poly (ether ether ketone) membrane: Effects of ACNT in improving polymer electrolyte fuel cell performance under reduced relative humidity. Composites Part B-Engineering 2020; 188: 107890. doi: 10.1016/j.compositesb.2020.107890 DOI: https://doi.org/10.1016/j.compositesb.2020.107890
14. He S, Ai Y, Dai W, et al. Composite membranes anchoring phosphotungstic acid by β-cyclodextrins modified halloysite nanotubes. Polymer Testing 2021; 100: 107246. doi: 10.1016/j.polymertesting.2021.107246 DOI: https://doi.org/10.1016/j.polymertesting.2021.107246
15. Liu X, Li Y, Xue J, et al. Magnetic field alignment of stable proton-conducting channels in an electrolyte membrane. Nature Communications 2019; 10(1): 842. doi: 10.1038/s41467-019-08622-2 DOI: https://doi.org/10.1038/s41467-019-08622-2
16. Liu X, Zhang J, Zheng C, et al. Oriented proton-conductive nano-sponge-facilitated polymer electrolyte membranes. Energy & Environmental Science 2020; 13(1): 297-309. doi: 10.1039/c9ee03301g DOI: https://doi.org/10.1039/C9EE03301G
17. He S, Liu S, Dai W, et al. Nanocomposite Proton Exchange Membranes Incorporating Phosphotungstic Acid Anchored on Imidazole-Functionalized Halloysite Nanotubes. Journal of the Electrochemical Society 2018; 165(11): F951-F958. doi: 10.1149/2.0601811jes DOI: https://doi.org/10.1149/2.0601811jes
18. Xu D, Zhang G, Zhang N, et al. Surface modification of heteropoly acid/SPEEK membranes by polypyrrole with a sandwich structure for direct methanol fuel cells. Journal of Materials Chemistry 2010; 20(41): 9239-9245. doi: 10.1039/c0jm02167a DOI: https://doi.org/10.1039/c0jm02167a
19. Lee H, Dellatore SM, Miller WM, et al. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007; 318(5849): 426-430. doi: 10.1126/science.1147241 DOI: https://doi.org/10.1126/science.1147241
20. Alfieri ML, Weil T, Ng DYW, et al. Polydopamine at biological interfaces. Advances in Colloid and Interface Science 2022; 305102689. doi: 10.1016/j.cis.2022.102689 DOI: https://doi.org/10.1016/j.cis.2022.102689
21. Liu T, Kim KC, Lee B, et al. Self-polymerized dopamine as an organic cathode for Li- and Na-ion batteries. Energy & Environmental Science 2017; 10(1): 205-215. doi: 10.1039/c6ee02641a DOI: https://doi.org/10.1039/C6EE02641A
22. Zhang C, Ou Y, Lei WX, et al. CuSO4/H2O2-Induced Rapid Deposition of Polydopamine Coatings with High Uniformity and Enhanced Stability. Angewandte Chemie-International Edition 2016; 55(9): 3054-3057. doi: 10.1002/anie.201510724 DOI: https://doi.org/10.1002/anie.201510724
23. Qi X, Huang Y, You S, et al. Engineering Robust Ag-Decorated Polydopamine Nano-Photothermal Platforms to Combat Bacterial Infection and Prompt Wound Healing. Advanced Science 2022; 9(11): 2106015. doi: 10.1002/advs.202106015 DOI: https://doi.org/10.1002/advs.202106015
24. Wang Z, Zou Y, Li Y, et al. Metal-Containing Polydopamine Nanomaterials: Catalysis, Energy, and Theranostics. Small 2020; 16(18): 1907042. doi: 10.1002/smll.201907042 DOI: https://doi.org/10.1002/smll.201907042
25. Liu Y, Ai K, Lu L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chemical Reviews 2014; 114(9): 5057-5115. doi: 10.1021/cr400407a DOI: https://doi.org/10.1021/cr400407a
26. He S, Dai W, Zhai S, et al. Sulfonated poly(ether ether ketone) composite membranes based on amino-modified halloysite nanotubes that effectively immobilize phosphotungstic acid. Journal of Polymer Science 2020; 58(18): 2625-2633. doi: 10.1002/pol.20200035 DOI: https://doi.org/10.1002/pol.20200035
27. Zhang Y, Wang H, Qian P, et al. Hybrid proton exchange membrane of sulfonated poly(ether ether ketone) containing polydopamine-coated carbon nanotubes loaded phosphotungstic acid for vanadium redox flow battery. Journal of Membrane Science 2021; 625: 119159. doi: 10.1016/j.memsci.2021.119159 DOI: https://doi.org/10.1016/j.memsci.2021.119159
28. Wei P, Huang D, Sui Y, et al. Polydopamine-coated polyimide nanofiber to anchor HPW and construct the pocket-like composite membrane with excellent proton conductivity and stability. International Journal of Hydrogen Energy 2023; 48(89): 34804-34815. doi: 10.1016/j.ijhydene.2023.05.284 DOI: https://doi.org/10.1016/j.ijhydene.2023.05.284
29. Gong C, Liu H, Zhang B, et al. High level of solid superacid coated poly(vinylidene fluoride) electrospun nanofiber composite polymer electrolyte membranes. Journal of Membrane Science 2017; 535: 113-121. doi: 10.1016/j.memsci.2017.04.037 DOI: https://doi.org/10.1016/j.memsci.2017.04.037
30. He S, Dai W, Yang W, et al. Nanocomposite proton exchange membranes based on phosphotungstic acid immobilized by polydopamine-coated halloysite nanotubes. Polymer Testing 2019; 73: 242-249. doi: 10.1016/j.polymertesting.2018.11.038 DOI: https://doi.org/10.1016/j.polymertesting.2018.11.038
31. Zhai S, Lu Z, Ai Y, et al. High performance nanocomposite proton exchange membranes based on the nanohybrids formed by chemically bonding phosphotungstic acid with covalent organic frameworks. Journal of Power Sources 2023; 554: 232332. doi: 10.1016/j.jpowsour.2022.232332 DOI: https://doi.org/10.1016/j.jpowsour.2022.232332
32. Zhang Z, Ren J, Xu J, et al. Long-term durable solid state electrolyte membranes based on a metal-organic framework with phosphotungstic acid confined in the mesoporous cages. International Journal of Hydrogen Energy 2020; 45(51): 27527-27538. doi: 10.1016/j.ijhydene.2020.07.024 DOI: https://doi.org/10.1016/j.ijhydene.2020.07.024
33. Feng M, Yu S, Wu P, et al. Rapid, high-efficient and selective removal of cationic dyes from wastewater using hollow polydopamine microcapsules: Isotherm, kinetics, thermodynamics and mechanism. Applied Surface Science 2021; 542: 148633. doi: 10.1016/j.apsusc.2020.148633 DOI: https://doi.org/10.1016/j.apsusc.2020.148633
34. Elanthamilan E, Ganeshkumar A, Wang SF, et al. Fabrication of polydopamine/polyaniline decorated multiwalled carbon nanotube composite as multifunctional electrode material for supercapacitor applications. Synthetic Metals 2023; 298: 117423. doi: 10.1016/j.synthmet.2023.117423 DOI: https://doi.org/10.1016/j.synthmet.2023.117423
35. Subudhi S, Mansingh S, Swain G, et al. HPW-Anchored UiO-66 Metal-Organic Framework: A Promising Photocatalyst Effective toward Tetracycline Hydrochloride Degradation and H2 Evolution via Z-Scheme Charge Dynamics. Inorganic Chemistry 2019; 58(8): 4921-4934. doi: 10.1021/acs.inorgchem.8b03544 DOI: https://doi.org/10.1021/acs.inorgchem.8b03544
36. Zhang Z, Ren J, Xu J, et al. Enhanced proton conductivity of sulfonated poly(arylene ether ketone sulfone) polymers by incorporating phosphotungstic acid-ionic-liquid-functionalized metal-organic framework. Journal of Membrane Science 2021; 630: 119304. doi: 10.1016/j.memsci.2021.119304 DOI: https://doi.org/10.1016/j.memsci.2021.119304
37. Ma Y, Li A, Wang C, et al. Preparation of HPW@UiO-66 catalyst with defects and its application in oxidative desulfurization. Chemical Engineering Journal 2021; 404: 127062. doi: 10.1016/j.cej.2020.127062 DOI: https://doi.org/10.1016/j.cej.2020.127062
38. Zhang J, He X, Yu S, et al. A novel dental adhesive containing Ag/polydopamine-modified HA fillers with both antibacterial and mineralization properties. Journal of Dentistry 2021; 111: 103710. doi: 10.1016/j.jdent.2021.103710 DOI: https://doi.org/10.1016/j.jdent.2021.103710
39. Zeng R, Deng H, Xiao Y, et al. Cross-linked graphene/carbon nanotube networks with polydopamine "glue" for flexible supercapacitors. Composites Communications 2018; 10: 73-80. doi: 10.1016/j.coco.2018.07.002 DOI: https://doi.org/10.1016/j.coco.2018.07.002
40. Huang X, Liu X. Morphology control of highly efficient visible-light driven carbon-doped POM photocatalysts. Applied Surface Science 2020; 505: 144527. doi: 10.1016/j.apsusc.2019.144527 DOI: https://doi.org/10.1016/j.apsusc.2019.144527
41. Zhai S, Song H, Jia X, et al. Fabrication of water-insoluble phosphotungstic acid-carbon nitride nanohybrids for promoting proton transport of nanocomposite proton exchange membranes. Journal of Power Sources 2021; 506: 230195. doi: 10.1016/j.jpowsour.2021.230195 DOI: https://doi.org/10.1016/j.jpowsour.2021.230195
42. Kim JS, Choi MC, Jeong KM, et al. Enhanced interaction in the polyimide/sepiolite hybrid films via acid activating and polydopamine coating of sepiolite. Polymers for Advanced Technologies 2018; 29(5): 1404-1413. doi: 10.1002/pat.4252 DOI: https://doi.org/10.1002/pat.4252
43. Zhang X, Ma H, Pei T, et al. Anchoring HPW by amino-modified MIL-101(Cr) to improve the properties of SPEEK in proton exchange membranes. Journal of Applied Polymer Science 2023; 140(25): 53978. doi: 10.1002/app.53978 DOI: https://doi.org/10.1002/app.53978
44. Dong C, Shi Z, Zhou Q. Preparation and investigation of acid-base composite membranes with modified graphitic carbon nanosheets for direct methanol fuel cells. Journal of Applied Polymer Science 2020; 137(45): 49388. doi: 10.1002/app.49388 DOI: https://doi.org/10.1002/app.49388
45. Wang X, Rong Y, Wang F, et al. High performance proton exchange membranes with double proton conduction pathways by introducing MOF impregnated with protic ionic liquid into SPEEK. Microporous and Mesoporous Materials 2022; 346: 112314. doi: 10.1016/j.micromeso.2022.112314 DOI: https://doi.org/10.1016/j.micromeso.2022.112314
46. Tsen W. Attapulgite solvent-free nanofluids modified SPEEK proton exchange membranes for direct methanol fuel cells. Ionics 2020; 26(11): 5651-5660. doi: 10.1007/s11581-020-03680-9 DOI: https://doi.org/10.1007/s11581-020-03680-9
47. Zhong F, Zeng Z, Liu Y, et al. Modification of sulfonated poly (etherether ketone) composite polymer electrolyte membranes with 2D molybdenum disulfide nanosheet-coated carbon nanotubes for direct methanol fuel cell application. Polymer 2022; 249: 124839. doi: 10.1016/j.polymer.2022.124839 DOI: https://doi.org/10.1016/j.polymer.2022.124839
48. Murmu R, Roy D, Patra SC, et al. Preparation and characterization of the SPEEK/PVA/Silica hybrid membrane for direct methanol fuel cell (DMFC). Polymer Bulletin 2022; 79(4): 2061-2087. doi: 10.1007/s00289-021-03602-3 DOI: https://doi.org/10.1007/s00289-021-03602-3
49. Zhu B, Sui Y, Wei P, et al. NH2-UiO-66 coated fibers to balance the excellent proton conduction efficiency and significant dimensional stability of proton exchange membrane. Journal of Membrane Science 2021; 628: 119214. doi: 10.1016/j.memsci.2021.119214 DOI: https://doi.org/10.1016/j.memsci.2021.119214
50. Zhang Y, Wang H, Yu W, et al. Sulfonated poly(ether ether ketone)-based hybrid membranes containing polydopamine-decorated multiwalled carbon nanotubes with acid-base pairs for all vanadium redox flow battery. Journal of Membrane Science 2018; 564: 916-925. doi: 10.1016/j.memsci.2018.07.065 DOI: https://doi.org/10.1016/j.memsci.2018.07.065
51. Zhai S, Jia X, Lu Z, et al. Highly ion selective composite proton exchange membranes for vanadium redox flow batteries by the incorporation of UiO-66-NH2 threaded with ion conducting polymers. Journal of Membrane Science 2022; 662: 121003. doi: 10.1016/j.memsci.2022.121003 DOI: https://doi.org/10.1016/j.memsci.2022.121003
52. Zhai S, Lu Z, Ai Y, et al. Highly selective proton exchange membranes for vanadium redox flow batteries enabled by the incorporation of water-insoluble phosphotungstic acid-metal organic framework nanohybrids. Journal of Membrane Science 2022; 645: 120214. doi: 10.1016/j.memsci.2021.120214 DOI: https://doi.org/10.1016/j.memsci.2021.120214
53. Bano S, Negi YS, Illathvalappil R, et al. Studies on nano composites of SPEEK/ethylene glycol/cellulose nanocrystals as promising proton exchange membranes. Electrochimica Acta 2019; 293: 260-272. doi: 10.1016/j.electacta.2018.10.029 DOI: https://doi.org/10.1016/j.electacta.2018.10.029
54. Wu J, Wang F, Fan X, et al. Phosphoric acid-doped Gemini quaternary ammonium-grafted SPEEK membranes with superhigh proton conductivity and mechanical strength for direct methanol fuel cells. Journal of Membrane Science 2023; 672: 121431. doi: 10.1016/j.memsci.2023.121431 DOI: https://doi.org/10.1016/j.memsci.2023.121431
55. Zhang Z, Liu H, Dong T, et al. Phosphonate poly(vinylbenzyl chloride)-Modified Sulfonated poly(aryl ether nitrile) for Blend Proton Exchange Membranes: Enhanced Mechanical and Electrochemical Properties. Polymers 2023; 15(15): 3203. doi: 10.3390/polym15153203 DOI: https://doi.org/10.3390/polym15153203
56. Xiao Y, Shen X, Sun R, et al. Polybenzimidazole membrane crosslinked with quaternized polyaniline as high-temperature proton exchange membrane: Enhanced proton conductivity and stability. Journal of Membrane Science 2022; 660: 120795. doi: 10.1016/j.memsci.2022.120795 DOI: https://doi.org/10.1016/j.memsci.2022.120795




.jpg)
.jpg)

