Ultrafast-adsorption-kinetics molecular sieving of propylene from propane
DOI:
https://doi.org/10.18686/cest.v2i2.126Abstract
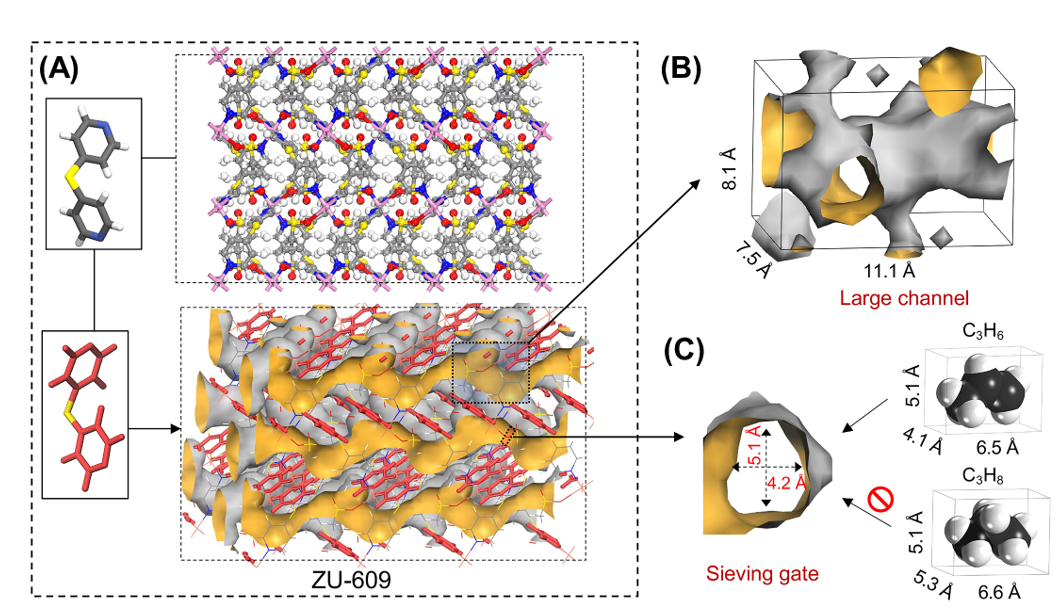
The separation of propylene (C3H6) and propane (C3H8) is very costly due to similar physical-chemical properties and has been listed as one of the seven chemical separations to change the world. High-purity C3H6 is an important raw material to produce polypropylene and acrylonitrile. However, C3H8 is produced as a by-product in the production process of C3H6, which has a similar structure and boiling point as those of C3H6. Traditionally, the separation of C3H6 and C3H8 by distillation has high energy consumption and an unremarkable separation effect. Therefore, there is an urgent need to develop more energy-saving and efficient methods for the separation of C3H6 and C3H8.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Ruike Zhang, Jiong Zhou

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
1. Sholl DS, Lively RP. Seven chemical separations to change the world. Nature. 2016; 532(7600): 435-437. doi: 10.1038/532435a DOI: https://doi.org/10.1038/532435a
2. Chen S, Chang X, Sun G, et al. Propane dehydrogenation: catalyst development, new chemistry, and emerging technologies. Chemical Society Reviews. 2021; 50(5): 3315-3354. doi: 10.1039/d0cs00814a DOI: https://doi.org/10.1039/D0CS00814A
3. Wu Y, Weckhuysen BM. Separation and Purification of Hydrocarbons with Porous Materials. Angewandte Chemie International Edition. 2021; 60(35): 18930-18949. doi: 10.1002/anie.202104318 DOI: https://doi.org/10.1002/anie.202104318
4. Liang B, Zhang X, Xie Y, et al. An Ultramicroporous Metal–Organic Framework for High Sieving Separation of Propylene from Propane. Journal of the American Chemical Society. 2020; 142(41): 17795-17801. doi: 10.1021/jacs.0c09466 DOI: https://doi.org/10.1021/jacs.0c09466
5. Wang L, Xue W, Zhu H, et al. Stepwise Engineering the Pore Aperture of a Cage‐like MOF for the Efficient Separation of Isomeric C4 Paraffins under Humid Conditions. Angewandte Chemie International Edition. 2023; 62(11). doi: 10.1002/anie.202218596 DOI: https://doi.org/10.1002/anie.202218596
6. Zheng J, Chen X, Ma J. Advances in solid adsorbent materials for direct air capture of CO2. Clean Energy Science and Technology. 2023; 1(2). doi: 10.18686/cest.v1i2.95 DOI: https://doi.org/10.18686/cest.v1i2.95
7. Li L, Guo L, Olson DH, et al. Discrimination of xylene isomers in a stacked coordination polymer. Science. 2022; 377(6603): 335-339. doi: 10.1126/science.abj7659 DOI: https://doi.org/10.1126/science.abj7659
8. Cui J, Zhang Z, Yang L, et al. A molecular sieve with ultrafast adsorption kinetics for propylene separation. Science. 2024; 383(6679): 179-183. doi: 10.1126/science.abn8418 DOI: https://doi.org/10.1126/science.abn8418
9. Yan M, Wang Y, Chen J, et al. Potential of nonporous adaptive crystals for hydrocarbon separation. Chemical Society Reviews. 2023; 52(17): 6075-6119. doi: 10.1039/d2cs00856d DOI: https://doi.org/10.1039/D2CS00856D
10. Yan M, Wang Y, Zhou J. Separation of toluene and alcohol azeotropes by nonporous adaptive crystals of pillar[n]arenes with analytical purity of 100%. Cell Reports Physical Science. 2023; 4(10): 101637. doi: 10.1016/j.xcrp.2023.101637 DOI: https://doi.org/10.1016/j.xcrp.2023.101637
11. Slater AG, Cooper AI. Function-led design of new porous materials. Science. 2015; 348(6238). doi: 10.1126/science.aaa8075 DOI: https://doi.org/10.1126/science.aaa8075




.jpg)
.jpg)

