Prussian blue nanoparticles: Synthesis and experimental evaluation as electrocatalyst for hydrogen evolution reaction
DOI:
https://doi.org/10.18686/cest.v2i3.121Keywords:
Prussian blue; electrocatalysis; energy conversion; HERAbstract
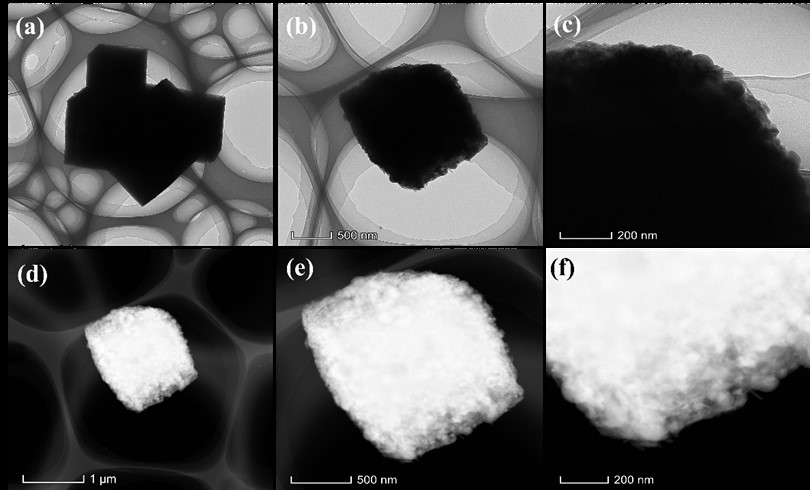
The reliance on fossil fuels has led to numerous environmental challenges, highlighting the urgent need for alternative energy sources that minimize contamination and promote eco-friendliness. In this context, hydrogen (H2) emerges as a promising fuel due to its zero-carbon emissions. Within various methods for H2 production, electrochemical water splitting (EWS) stands out as a viable approach. Traditionally, noble metals, such as platinum and iridium, have been employed as electrocatalysts to efficiently facilitate the hydrogen evolution reaction (HER) in desired electrolytes (such as alkaline). Recently, research has focused on the use of Prussian blue (PB) as an innovative electrocatalyst material for EWS. Herein, we developed PB-based electrocatalysts for HER in an alkaline medium. The electrocatalyst comprising PB combined with phosphorus exhibited impressive electrochemical properties, achieving a minimal overpotential of 103 mV at a current density of 10 mA/cm2 and maintaining durability over 20 h, along with extended electrochemical performance. This material composition has considerable promise as a potential option for energy conversion systems, which can aid future sustainability initiatives.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Mukhtiar Ahmed, Irfan Ali Soomro, Kishore Chand, Yang Yang

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
1. Chen L, Ren J, Yuan Z. Design strategies of phosphorus-containing catalysts for photocatalytic, photoelectrochemical and electrocatalytic water splitting. Green Chemistry. 2022; 24(2): 713-747. doi: 10.1039/D1GC03768D DOI: https://doi.org/10.1039/D1GC03768D
2. Aftab U, Tahira A, Samo AH, et al. Mixed CoS2@Co3O4 composite material: An efficient nonprecious electrocatalyst for hydrogen evolution reaction. International Journal of Hydrogen Energy. 2020; 45(27): 13805-13813. doi: 10.1016/j.ijhydene.2020.03.131 DOI: https://doi.org/10.1016/j.ijhydene.2020.03.131
3. Hanan A, Shu D, Aftab U, et al. Co2FeO4@ rGO composite: Towards trifunctional water splitting in alkaline media. International Journal of Hydrogen Energy. 2022; 47(80): 33919-33937. doi: 10.1016/j.ijhydene.2022.07.269 DOI: https://doi.org/10.1016/j.ijhydene.2022.07.269
4. Weng C, Lv X, Ren J, et al. Engineering Gas–Solid–Liquid Triple-Phase Interfaces for Electrochemical Energy Conversion Reactions. Electrochemical Energy Reviews. 2022; 5(Suppl 1): 19. doi: 10.1007/s41918-022-00133-x DOI: https://doi.org/10.1007/s41918-022-00133-x
5. Laghari AJ, Aftab U, Tahira A, et al. MgO as promoter for electrocatalytic activities of Co3O4–MgO composite via abundant oxygen vacancies and Co2+ ions towards oxygen evolution reaction. International Journal of Hydrogen Energy. 2023; 48(34): 12672-12682. doi: 10.1016/j.ijhydene.2022.04.169 DOI: https://doi.org/10.1016/j.ijhydene.2022.04.169
6. Ali Bhutto Y, Pandey AK, Saidur R, et al. Electrical and thermal performance assessment of photovoltaic thermal system integrated with organic phase change material. E3S Web of Conferences. 2024; 488: 01007. doi: 10.1051/e3sconf/202448801007 DOI: https://doi.org/10.1051/e3sconf/202448801007
7. Hanan A, Lakhan MN, Shu D, et al. An efficient and durable bifunctional electrocatalyst based on PdO and Co2FeO4 for HER and OER. International Journal of Hydrogen Energy. 2023; 48(51): 19494-19508. doi: 10.1016/j.ijhydene.2023.02.049 DOI: https://doi.org/10.1016/j.ijhydene.2023.02.049
8. Aftab U, Solangi MY, Tahira A, et al. An advanced PdNPs@MoS2 nanocomposite for efficient oxygen evolution reaction in alkaline media. RSC Advances. 2023; 13(46): 32413-32423. doi: 10.1039/D3RA04738E DOI: https://doi.org/10.1039/D3RA04738E
9. Raja Sulaiman RR, Hanan A, Wong WY, et al. Structurally modified MXenes-based catalysts for application in hydrogen evolution reaction: A review. Catalysts. 2022; 12(12): 1576. doi: 10.3390/catal12121576 DOI: https://doi.org/10.3390/catal12121576
10. Ibupoto ZH, Tahira A, Shah AA, et al. NiCo2O4 nanostructures loaded onto pencil graphite rod: An advanced composite material for oxygen evolution reaction. International Journal of Hydrogen Energy. 2022; 47(10): 6650-6665. doi: 10.1016/j.ijhydene.2021.12.024 DOI: https://doi.org/10.1016/j.ijhydene.2021.12.024
11. Hao LP, Hanan A, Walvekar R, et al. Synergistic Integration of MXene and Metal-Organic Frameworks for Enhanced Electrocatalytic Hydrogen Evolution in an Alkaline Environment. Catalysts. 2023; 13(5): 802. doi: 10.3390/catal13050802 DOI: https://doi.org/10.3390/catal13050802
12. Zuo Y, Bellani S, Saleh G, et al. Ru–Cu Nanoheterostructures for Efficient Hydrogen Evolution Reaction in Alkaline Water Electrolyzers. Journal of the American Chemical Society. 2023; 145(39): 21419-21431. doi: 10.1021/jacs.3c06726 DOI: https://doi.org/10.1021/jacs.3c06726
13. Ren J, Chen L, Wang H, et al. Water electrolysis for hydrogen production: From hybrid systems to self-powered/catalyzed devices. Energy & Environmental Science. 2024; 17(1): 49-113. doi: 10.1039/D3EE02467A DOI: https://doi.org/10.1039/D3EE02467A
14. Hanan A, Ahmed M, Lakhan MN, et al. Novel rGO@Fe3O4 nanostructures: An active electrocatalyst for hydrogen evolution reaction in alkaline media. Journal of the Indian Chemical Society. 2022; 99(5): 100442. doi: 10.1016/j.jics.2022.100442 DOI: https://doi.org/10.1016/j.jics.2022.100442
15. Qureshi RA, Hanan A, Abro MI, et al. Facile eggplant assisted mixed metal oxide nanostructures: A promising electrocatalyst for water oxidation in alkaline media. Materials Today Sustainability. 2023; 23: 100446. doi: 10.1016/j.mtsust.2023.100446 DOI: https://doi.org/10.1016/j.mtsust.2023.100446
16. Balu S, Hanan A, Venkatesvaran H, et al. Recent Progress in Surface-Defect Engineering Strategies for Electrocatalysts toward Electrochemical CO2 Reduction: A Review. Catalysts. 2023; 13(2): 393. doi: 10.3390/catal13020393 DOI: https://doi.org/10.3390/catal13020393
17. Ren J, Chen L, Wang H, Yuan Z. High-entropy alloys in electrocatalysis: From fundamentals to applications. Chemical Society Reviews. 2023; 52(23): 8319-8373. doi: 10.1039/D3CS00557G DOI: https://doi.org/10.1039/D3CS00557G
18. Shaheen I, Ali I, Bibi F, et al. Integrating 1D/2D nanostructure based on Ni–Co-oxalate for energy storage applications. Ceramics International. 2023; 50(7A): 10789-10796. doi: 10.1016/j.ceramint.2023.12.394 DOI: https://doi.org/10.1016/j.ceramint.2023.12.394
19. Chen J, Wei L, Mahmood A, et al. Prussian blue, its analogues and their derived materials for electrochemical energy storage and conversion. Energy Storage Materials. 2020; 25: 585-612. doi: 10.1016/j.ensm.2019.09.024 DOI: https://doi.org/10.1016/j.ensm.2019.09.024
20. Guichard C, Le Hô AS, Williams H. Prussian Blue: Chemistry, Commerce, and Colour in Eighteenth-Century Paris. Art History. 2023; 46(1): 154-186. doi: 10.1111/1467-8365.12695 DOI: https://doi.org/10.1111/1467-8365.12695
21. Zhao D, Lu Y, Ma D. Effects of Structure and Constituent of Prussian Blue Analogs on Their Application in Oxygen Evolution Reaction. Molecules. 2020; 25(10): 2304. doi: 10.3390/molecules25102304 DOI: https://doi.org/10.3390/molecules25102304
22. Bhutto YA, Pandey AK, Saidur R, et al. Analyzing the thermal potential of binary 2D (h-BN/Gr) nanoparticles enhanced lauric acid phase change material for photovoltaic thermal system application. Journal of Energy Storage. 2023; 73: 109116. doi: 10.1016/j.est.2023.109116 DOI: https://doi.org/10.1016/j.est.2023.109116
23. Fayaz M, Lai W, Li J, et al. Prussian blue analogues and their derived materials for electrochemical energy storage: Promises and Challenges. Materials Research Bulletin. 2024; 170: 112593. doi: 10.1016/j.materresbull.2023.112593 DOI: https://doi.org/10.1016/j.materresbull.2023.112593
24. Samo A, Aftab U, Cao D, et al. Schematic synthesis of cobalt-oxide (Co3O4) supported cobalt-sulfide (CoS) composite for oxygen evolution reaction. Digest Journal of Nanomaterials & Biostructures (DJNB). 2022; 17(1): 109. doi: 10.15251/djnb.2022.171.109 DOI: https://doi.org/10.15251/DJNB.2022.171.109
25. Solangi MY, Aftab U, Tahira A, et al. In-situ growth of nonstoichiometric CrO0.87 and Co3O4 hybrid system for the enhanced electrocatalytic water splitting in alkaline media. International Journal of Hydrogen Energy. 2023; 48(93): 36439-36451. doi: 10.1016/j.ijhydene.2023.06.059 DOI: https://doi.org/10.1016/j.ijhydene.2023.06.059
26. Jia N, Huang B, Chen L, et al. A simple non-enzymatic hydrogen peroxide sensor using gold nanoparticles-graphene-chitosan modified electrode. Sensors and Actuators B: Chemical. 2014; 195: 165-170. doi: 10.1016/j.snb.2014.01.043 DOI: https://doi.org/10.1016/j.snb.2014.01.043
27. Lu Y, Saroja APVK, Wei R, Xu Y. Engineering metal selenides for sodium-and potassium-ion batteries. Cell Reports Physical Science. 2021; 2(9): 100555. doi: 10.1016/j.xcrp.2021.100555 DOI: https://doi.org/10.1016/j.xcrp.2021.100555
28. Buser HJ, Schwarzenbach D, Petter W, Ludi A. The crystal structure of Prussian blue: Fe4[Fe(CN)6]3·xH2O. Inorganic Chemistry. 1977; 16(11): 2704-2710. doi: 10.1021/ic50177a008 DOI: https://doi.org/10.1021/ic50177a008
29. Hussain I, Ahmad M, Kewate OJ, et al. V-MXenes for energy storage/conversion applications. ChemSusChem. 2024; 17(15): e202400283. doi: 10.1002/cssc.202400283 DOI: https://doi.org/10.1002/cssc.202400283
30. Hanan A, Laghari AJ, Solangi MY, et al. CdO/Co3O4 nanocomposite as an efficient electrocatalyst for oxygen evolution reaction in alkaline media. International Journal of Engineering Science Technologies. 2022; 6(1): 1-10. doi: 10.29121/IJOEST.v6.i1.2022.259 DOI: https://doi.org/10.29121/ijoest.v6.i1.2022.259
31. Hanan A, Lakhan MN, Solangi MY, et al. MXene based electrocatalyst: CoS2@Ti3C2Tx composite for hydrogen evolution reaction in alkaline media. Materials Today Sustainability. 2023; 24: 100585. doi: 10.1016/j.mtsust.2023.100585 DOI: https://doi.org/10.1016/j.mtsust.2023.100585
32. Ahmed M, Hanan A, Lakhan MN, et al. One-pot synthesis of crystalline structure: Nickel-iron phosphide and selenide for hydrogen production in alkaline water splitting. Journal of Electrochemical Science and Engineering. 2023; 13(3): 575-588. doi: 10.5599/jese.1721 DOI: https://doi.org/10.5599/jese.1721
33. Ahmed M, Lakhan MN, Shar AH, et al. Electrochemical performance of grown layer of Ni(OH)2 on nickel foam and treatment with phosphide and selenide for efficient water splitting. Journal of the Indian Chemical Society. 2022; 99(1): 100281. doi: 10.1016/j.jics.2021.100281 DOI: https://doi.org/10.1016/j.jics.2021.100281
34. Solangi MY, Samo AH, Laghari AJ, et al. MnO2@Co3O4 nanocomposite based electrocatalyst for effective oxygen evolution reaction. Sukkur IBA Journal of Emerging Technologies. 2022; 5(1): 32-40. doi: 10.30537/sjet.v5i1.958 DOI: https://doi.org/10.30537/sjet.v5i1.958
35. Lakhan MN, Hanan A, Wang Y, et al. Recent Progress on Nickel-and Iron-Based Metallic Organic Frameworks for Oxygen Evolution Reaction: A Review. Langmuir. 2024; 40(5): 2465-2486. doi: 10.1021/acs.langmuir.3c03558 DOI: https://doi.org/10.1021/acs.langmuir.3c03558
36. Hanan A, Lakhan MN, Bibi F, et al. MOFs coupled transition metals, graphene, and MXenes: Emerging electrocatalysts for hydrogen evolution reaction. Chemical Engineering Journal. 2024; 482: 148776. doi: 10.1016/j.cej.2024.148776 DOI: https://doi.org/10.1016/j.cej.2024.148776
37. Hanan A. Magnesium doped cobalt-oxide composite for active oxygen evolution reaction. Journal of Applied and Emerging Sciences. 2021; 11(2): 210-216. doi: 10.36785/JAES.112519
38. Deng BL, Guo LP, Lu Y, et al. Sulfur–nitrogen co-doped graphene supported cobalt–nickel sulfide rGO@SN-CoNi2S4 as highly efficient bifunctional catalysts for hydrogen/oxygen evolution reactions. Rare Metals. 2022; 41(3): 911-920. doi: 10.1007/s12598-021-01828-8 DOI: https://doi.org/10.1007/s12598-021-01828-8
39. Borthakur P, Boruah PK, Das MR, et al. CoS2 Nanoparticles Supported on rGO, g-C3N4, BCN, MoS2, and WS2 Two-Dimensional Nanosheets with Excellent Electrocatalytic Performance for Overall Water Splitting: Electrochemical Studies and DFT Calculations. ACS Applied Energy Materials. 2021; 4(2): 1269-1285. doi: 10.1021/acsaem.0c02509 DOI: https://doi.org/10.1021/acsaem.0c02509
40. Zhu H, Zhang J, Yanzhang R, et al. When Cubic Cobalt Sulfide Meets Layered Molybdenum Disulfide: A Core–Shell System Toward Synergetic Electrocatalytic Water Splitting. Advanced Materials. 2015; 27(32): 4752-4759. doi: 10.1002/adma.201501969 DOI: https://doi.org/10.1002/adma.201501969
41. Wang N, Li L, Zhao D, et al. Graphene Composites with Cobalt Sulfide: Efficient Trifunctional Electrocatalysts for Oxygen Reversible Catalysis and Hydrogen Production in the Same Electrolyte. Small. 2017; 13(33): 1701025. doi: 10.1002/smll.201701025 DOI: https://doi.org/10.1002/smll.201701025
42. Zahra R, Pervaiz E, Baig MM, et al. Three-dimensional hierarchical flowers-like cobalt-nickel sulfide constructed on graphitic carbon nitride: Bifunctional non-noble electrocatalyst for overall water splitting. Electrochimica Acta. 2022; 418: 140346. doi: 10.1016/j.electacta.2022.140346 DOI: https://doi.org/10.1016/j.electacta.2022.140346
43. Huang L, Wu H, Liu H, et al. Phosphorous doped cobalt-iron sulfide/carbon nanotube as active and robust electrocatalysts for water splitting. Electrochimica Acta. 2019; 318: 892-900. doi: 10.1016/j.electacta.2019.06.096Get DOI: https://doi.org/10.1016/j.electacta.2019.06.096
44. Liu Q, Pu Z, Asiri AM, et al. Nitrogen-doped carbon nanotube supported iron phosphide nanocomposites for highly active electrocatalysis of the hydrogen evolution reaction. Electrochimica Acta. 2014; 149: 324-329. doi: 10.1016/j.electacta.2014.10.105 DOI: https://doi.org/10.1016/j.electacta.2014.10.105
45. Chen WF, Wang CH, Sasaki K, et al. Highly active and durable nanostructured molybdenum carbide electrocatalysts for hydrogen production. Energy & Environmental Science. 2013; 6(3): 943-951. doi: 10.1039/C2EE23891H DOI: https://doi.org/10.1039/c2ee23891h
46. Chen WF, Sasaki K, Ma C, et al. Hydrogen-Evolution Catalysts Based on Non-Noble Metal Nickel–Molybdenum Nitride Nanosheets. Angewandte Chemie International Edition. 2012; 51(25): 6131-6135. doi: 10.1002/anie.201200699 DOI: https://doi.org/10.1002/anie.201200699




.jpg)
.jpg)

