Biochemical role of experimentally induced malnutrition on depressed brain and moderate mood with blood picture in chicken
DOI:
https://doi.org/10.18686/fnc.v1i2.48Keywords:
chicken; malnutrition; brain functions; blood indices; anemia; CBCAbstract
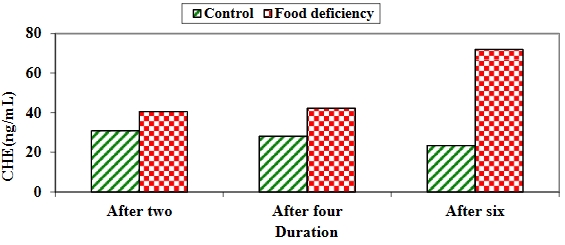
Background: Nutritional deficiency and malnutrition induce cyclothymia, depressed mood, and anemia. Therefore, in the present study, we experimentally induced malnutrition in chickens to follow up on brain functions and anemia profiles (blood indices) in chickens. Material and methods: The study was conducted on 60 one-day-old chicks that were equally divided into two groups and fed two different diets for six weeks. The control group was fed commercial grower and finisher rations during the growing and finishing periods, while the second group, which was the food deficiency test group, was fed yellow corn constantly during the growing and finishing periods. All chicks were weighed weekly to record weight differences. Whole blood samples and brain homogenates were collected from 10 chicks in each group every two weeks to evaluate brain tissue homogenate parameters, differential leukocyte count, complete blood count, and blood indices. Results: In the food deficiency group, acetylcholinesterase (ACHE) and total antioxidant capacity (TAC) consistently increased throughout the study period. Tumor necrosis factor-alpha (TNF-α) increased after the second and fourth weeks but showed no significant difference after the sixth week. Glutathione peroxidase (GPx) and superoxide dismutase (SOD) increased significantly in the control group during the entire experiment. In terms of blood counts, the white blood cells (WBCs) were consistently higher in the food deficiency group. Eosinophils were significantly elevated after two and four weeks but not after six weeks. Lymphocytes were elevated in the control group after the second week and in the food deficiency group after six weeks. Band neutrophils increased significantly in the food deficiency group after six weeks, while monocytes increased after two weeks in the food deficiency group and after six weeks in the control group. Basophils and segmented neutrophils increased after two weeks in the food deficiency group but showed no significant differences after four and six weeks in either group. Furthermore, hemoglobin (Hb), red blood cells (RBCs), packed cell volume (PCV), mean corpuscular volume (MCV), and platelet count increased significantly in the control group throughout the study. Mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) increased significantly after four and six weeks in the control group. Conclusion: Malnutrition can have high effects on brain functions and blood parameters.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Alaa Mohammed, Afaf Desouky, Raafat R. Mohammed, Hussein Abdel-Maksoud

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
1. Getawa S, Getaneh Z, Melku M. Hematological abnormalities and associated factors among undernourished under-five children attending University of Gondar Specialized Referral Hospital, northwest Ethiopia. Journal of Blood Medicine 2020; 11: 465–478. doi: 10.2147/JBM.S284572
2. Espinoza M, Perelli J, Olmos R, et al. Nutritional assessment as predictor of complications after hematopoietic stem cell transplantation. Revista Brasileira de Hematologia e Hemoterapia 2016; 38(1): 7–14. doi: 10.1016/j.bjhh.2015.10.002
3. Mansour HM. Nutrition and brain functions in health and disease. In: Mohamed W, Kobeissy F (editors). Nutrition and Psychiatric Disorders. Springer; 2022. pp. 3–26.
4. Favela LH, Martin J. “Cognition” and dynamical cognitive science. Minds and Machines 2017; 27(2): 331–355. doi: 10.1007/s11023-016-9411-4
5. Okon-Singer H, Hendler T, Pessoa L, Shackman AJ. The neurobiology of emotion-cognition interactions: Fundamental questions and strategies for future research. Frontiers in Human Neuroscience 2015; 9: 58. doi: 10.3389/fnhum.2015.00058
6. Galbiati A, d’Adda di Fagagna F. DNA damage in situ ligation followed by proximity ligation assay (DI-PLA). Methods in Molecular Biology 2019; 1896: 13–20. doi: 10.1007/978-1-4939-8931-7_2
7. Ellman GL, Courtney KD, Andres V Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology 1961; 7(2): 88–95. doi: 10.1016/0006-2952(61)90145-9
8. Azhar S, Cao L, Reaven E. Alteration of the adrenal antioxidant defense system during aging in rats. The Journal of Clinical Investigation 1995; 96(3): 1414–1424. doi: 10.1172/JCI118177
9. Petrovas C, Daskas SM, Lianidou ES. Determination of tumor necrosis factor-alpha (TNF-alpha) in serum by a highly sensitive enzyme amplified lanthanide luminescence immunoassay. Clinical Biochemistry 1999; 32(4): 241–247. doi: 10.1016/s0009-9120(99)00004-1
10. Nebot C, Moutet M, Huet P, et al. Spectrophotometric Assay of SOD activity based on the activated auto-oxidation of tetracyclic catechol. Analytical Biochemistry 1993; 214(2): 442–451. doi: 10.1006/abio.1993.1521
11. Nelly A, J.Randall S, Ivan SP, Harvey JC. Partial sequences of human plasma glutathione peroxidase and immunologic identification of milk glutathione peroxidase as the plasma enzyme. The Journal of Nutrition 1991; 121(8): 1243–1249. doi: 10.1093/jn/121.8.1243
12. Steel RGD, Torrie JH, Dickey DA. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed. McGraw-Hill; 1997.
13. Zielińska M, Łuszczki E, Dereń K. Dietary nutrient deficiencies and risk of depression (Review article 2018–2023). Nutrients 2023; 15(11): 2433. doi: 10.3390/nu15112433
14. Huang Q, Liu H, Suzuki K, et al. Linking What we eat to our mood: A review of diet, dietary antioxidants, and depression. Antioxidants 2019; 8(9): 376. doi: 10.3390/antiox8090376
15. Winick M, Noble A. Cellular response in rats during malnutrition at various ages. The Journal of Nutrition 1966; 89(3): 300–306. doi: 10.1093/jn/89.3.300
16. Crnic LS. Effects of nutrition and environment on brain biochemistry and behavior. Development Psychobiology 1983; 16(2): 129–145. doi: 10.1002/dev.420160206
17. Zivkovic AR, Paul GM, Hofer S, et al. Increased enzymatic activity of acetylcholinesterase indicates the severity of the sterile inflammation and predicts patient outcome following traumatic injury. Biomolecules 2023; 13(2): 267. doi: 10.3390/biom13020267
18. Nizri E, Hamra‐Amitay Y, Sicsic C, et al. Anti‐inflammatory properties of cholinergic up‐regulation: A new role for acetylcholinesterase inhibitors. Neuropharmacology 2006; 50(5): 540–547. doi: 10.1016/j.neuropharm.2005.10.013
19. Rosas‐Ballina M, Olofsson PS, Ochani M, et al. Acetylcholine‐synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011; 334(6052): 98–101. doi: 10.1126/science.1209985
20. Ben‐David Y, Kagan S, Cohen Ben‐Ami H, et al. RIC3, the cholinergic anti‐inflammatory pathway, and neuroinflammation. International Immunopharmacol 2020; 83: 106381. doi: 10.1016/j.intimp.2020.106381
21. Prenesti E, Berto S, Gosmaro F, et al. Dysmetabolisms can affect total antioxidant capacity (TAC) of human plasma: Determination of reference intervals of TAC by way of CUPRAC-BCS method. Antioxidants 2021; 10(1): 58. doi: 10.3390/antiox10010058
22. Feoli AM, Siqueira IR, Almeida L, et al. Effects of protein malnutrition on oxidative status in rat brain. Nutrition 2006; 22(2): 160–165. doi: 10.1016/j.nut.2005.06.007
23. Azevedo ZM, Luz RA, Victal SH, et al. Increased production of tumor necrosis factor-alpha in whole blood cultures from children with primary malnutrition. Brazilian Journal of Medical and Biological Research 2005; 38(2): 171–183. doi: 10.1590/s0100-879x2005000200005
24. Feuerstein GZ, Liu T, Barone FC. Cytokines, inflammation, and brain injury: Role of tumor necrosis factor-alpha. Cerebrovascular and Brain Metabolism Reviews 1994; 6(4): 341–360.
25. Arvin B, Neville LF, Barone FC, Feuerstein GZ. The role of inflammation and cytokines in brain injury. Neuroscience & Biobehavioral Reviews 1996; 20(3): 445–452. doi: 10.1016/0149-7634(95)00026-7
26. Kochanek PM, Hallenbeck JM. Polymorphonuclear leukocytes and monocytes/macrophages in the pathogenesis of cerebral ischemia and stroke. Stroke 1992; 23: 1367–1379. doi: 10.1161/01.STR.23.9.1367
27. Postal M, Lapa AT, Sinicato NA, et al. Depressive symptoms are associated with tumor necrosis factor alpha in systemic lupus erythematosus. Journal of Neuroinflammation 2016; 13(5). doi: 10.1186/s12974-015-0471-9
28. Himmerich H. Activity of the TNF-α system in patients with brain disorders and during psychopharmacological treatment. Current Pharmaceutical Analysis 2007; 3(1): 1–5. doi: 10.2174/157341207779802412
29. Matés JM, Pérez-Gómez C, Núñez de Castro I, et al. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. The International Journal of Biochemistry & Cell Biology 2002; 34(5): 439–458. doi: 10.1016/s1357-2725(01)00143-1
30. Olalla L, Aledo JC, Bannenberg G, Márquez J. The C-terminus of human glutaminase L mediates association with PDZ domain-containing proteins. FEBS Letters 2001; 488(3): 116–122. doi: 10.1016/s0014-5793(00)02373-5
31. Halliwell B. Oxygen radicals as key mediators in neurological disease: Fact or fiction? Annals of Neurology 1992; 32(S1): S10–S15. doi: 10.1002/ana.410320704
32. Arya AK, Kumar P, Midha Tanu, Singh M. Hematological profile of children with severe acute malnutrition: A tertiary care centre experience. International Journal of Contemporary Pediatrics 2017; 4(5): 1577–1580. doi: 10.18203/2349-3291.ijcp20173072
33. Khan S, Rubab Z, Hussain S, et al. Hematological profile of children with severe acute malnutrition at the Tertiary care hospital in Multan. Isra Medical Journal 2020; 12(1): 12–16.
34. Gohain EK, Pathak K, Choudhury B. A case control study of hematological changes in children with protein energy malnutrition attending Gauhati medical college and hospital. IOSR Journal of Dental and Medical Sciences 2016; 15(10): 25–29. doi: 10.9790/0853-1510012529
35. Jordan S, Tung N, Casanova-Acebes M, et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell 2019; 178(5): 1102–1114. doi: 10.1016/j.cell.2019.07.050
36. Corware K, Yardley V, Mack C, et al. Protein energy malnutrition increases arginase activity in monocytes and macrophages. Nutrition & Metabolism 2014; 11(51). doi: 10.1186/1743-7075-11-51
37. Takele Y, Adem E, Getahun M, et al. Malnutrition in healthy individuals results in increased mixed cytokine profiles, altered neutrophil subsets and function. PLoS One 2016; 11(8): e0157919. doi: 10.1371/journal.pone.0157919
38. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. The Journal of Gerontology: Series A 2014; 69(Suppl 1): S4–S9. doi: 10.1093/gerona/glu057
39. Tigner A, Ibrahim SA, Murray IV. Histology, white blood cell. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563148/ (accessed on 26 September 2023).




.jpg)
.jpg)

