Natural lutein from microalgae for aquaculture/mariculture: Benefits and mechanisms of action
DOI:
https://doi.org/10.18686/fnc304Keywords:
lutein; astaxanthin; mariculture; aquaculture; mitochondrial biogenesis; carotenoid esterification; microalgaeAbstract
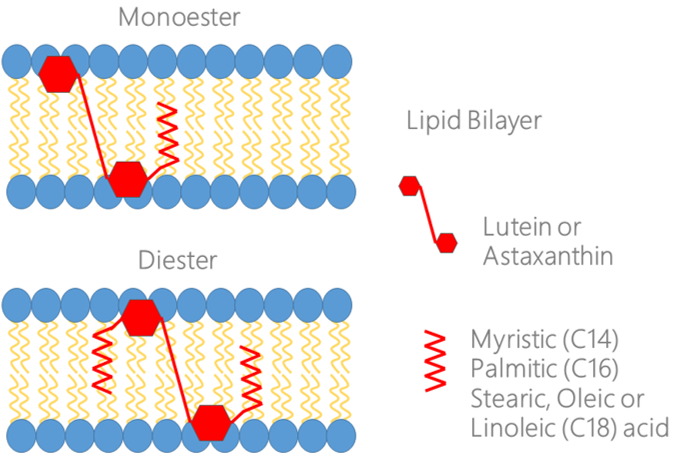
Lutein is a widespread carotenoid in the world, which is characterized by remarkable antioxidant activity. The impact of lutein for the treatment of chronic inflammation disorders makes it an important supplement for the support of active human longevity. Lutein can inhibit the proinflammatory NF-kappaB pathway, Nox-enzyme and VEGF-related pathologies. Despite the lack of a provitamin activity, it is often denoted as “the eye vitamin”. The main aim of this mini-review is an analysis of possible mechanisms of lutein effects focusing on their superior antioxidative/“vitamin” role in human health and aquaculture/mariculture. Recent publications clearly demonstrate the ability of lutein to stimulate mitochondrial biogenesis in both neuronal and muscle cells. It stimulates the tissue respiration and, most likely, it explains the accelerated nervous system development in neonatal children treated with lutein and lutein benefits for aqua/mariculture. Comparisons of characteristics of natural lutein and natural/synthetic astaxanthin included in this review allow the evaluation of their beneficial potentials. One of the most important advantages of natural vs. artificial substances is associated with natural carotenoid esterification and secondary structures. A comparative study of natural lutein and astaxanthin generation techniques illustrates the need for the development of new methods for fast and massive natural carotenoid production. Thus, the optimization of natural lutein production in cultivated microalgae may be considered as an important option in the further development of the carotenoid industry. All the important updates mentioned above for natural lutein will be summarized in this review.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Author(s)

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
1. Raghuvanshi S, Reed V, Blaner WS, et al. Cellular localization of β-carotene 15,15′ oxygenase-1 (BCO1) and β-carotene 9′,10′ oxygenase-2 (BCO2) in rat liver and intestine. Archives of biochemistry and biophysics. 2015; 572: 19–27. doi: 10.1016/j.abb.2014.12.024 DOI: https://doi.org/10.1016/j.abb.2014.12.024
2. Kim YK, Zuccaro MV, Costabile BK, et al. Tissue- and sex-specific effects of β-carotene 15,15′ oxygenase (BCO1) on retinoid and lipid metabolism in adult and developing mice. Archives of Biochemistry and Biophysics. 2015; 572: 11-18. doi: 10.1016/j.abb.2015.01.002 DOI: https://doi.org/10.1016/j.abb.2015.01.002
3. Madaro A, Torrissen O, Whatmore P, et al. Red and White Chinook Salmon (Oncorhynchus tshawytscha): Differences in the Transcriptome Profile of Muscle, Liver, and Pylorus. Marine Biotechnology. 2020; 22(4): 581-593. doi: 10.1007/s10126-020-09980-5 DOI: https://doi.org/10.1007/s10126-020-09980-5
4. Christiansen R, Lie O, Torrissen OJ. Growth and survival of Atlantic salmon, Salmo salar L., fed different dietary levels of astaxanthin. First-feeding fry. Aquaculture Nutrition. 1995; 1(3): 189–198. doi: 10.1111/j.1365-2095.1995.tb00043.x DOI: https://doi.org/10.1111/j.1365-2095.1995.tb00043.x
5. Cristaldi M, Anfuso CD, Spampinato G, et al. Comparative Efficiency of Lutein and Astaxanthin in the Protection of Human Corneal Epithelial Cells In Vitro from Blue-Violet Light Photo-Oxidative Damage. Applied Sciences. 2022; 12(3): 1268. doi: 10.3390/app12031268 DOI: https://doi.org/10.3390/app12031268
6. Ettefaghdoost M, Haghighi H. Impact of different dietary lutein levels on growth performance, biochemical and immuno-physiological parameters of oriental river prawn (Macrobrachium nipponense). Fish & Shellfish Immunology. 2021; 115: 86-94. doi: 10.1016/j.fsi.2021.05.024 DOI: https://doi.org/10.1016/j.fsi.2021.05.024
7. Talebi M, Khara H, Zorriehzahra M, et al. The Effects of Lutein on Growth and Blood Factors of Rainbow Trout. In: Proceedings of International Conference on Chemical, Ecology and Environmental Sciences; Pattaya, Thailand.
8. Yousefi M, Adineh H, Ghafarifarsani H, et al. Immunological, Antioxidant, Growth Responses, and Disease Resistance of Rainbow Trout, Oncorhynchus mykiss, with Feeding Diets Supplemented with Lactobacillus salivarius and Lutein. Annals of Animal Science. 2024; 24(4): 1211-1222. doi: 10.2478/aoas-2024-0033 DOI: https://doi.org/10.2478/aoas-2024-0033
9. Gazzolo D, Picone S, Gaiero A, et al. Early Pediatric Benefit of Lutein for Maturing Eyes and Brain—An Overview. Nutrients. 2021; 13(9): 3239. doi: 10.3390/nu13093239 DOI: https://doi.org/10.3390/nu13093239
10. Ingkasupart P, Manochai B, Song WT, et al. Antioxidant activities and lutein content of 11 marigold cultivars (Tagetes spp.) grown in Thailand. Food Science and Technology (Campinas). 2015; 35(2): 380-385. doi: 10.1590/1678-457x.6663 DOI: https://doi.org/10.1590/1678-457X.6663
11. Hamułka J, Koczara J, Gronek M. Lutein Content of Selected Polish Foods and Estimation of its Intake. Polish Journal of Food and Nutrition Sciences. 2005; 55(2): 201–206.
12. Lashmanova KA, Kuzivanova OA, Dymova OV. Northern berries as a source of carotenoids. Acta Biochimica Polonica. 2012; 59(1): 133-134. DOI: https://doi.org/10.18388/abp.2012_2188
13. Montuori E, Lima S, Marchese A, et al. Lutein Production and Extraction from Microalgae: Recent Insights and Bioactive Potential. International Journal of Molecular Sciences. 2024; 25(5): 2892. doi: 10.3390/ijms25052892 DOI: https://doi.org/10.3390/ijms25052892
14. Šivel M, Kráčmar S, Fišera M, et al. Lutein content in marigold flower (Tagetes erecta L.) concentrates used for production of food supplements. Czech Journal of Food Sciences. 2014; 32(6): 521-525. doi: 10.17221/104/2014-cjfs DOI: https://doi.org/10.17221/104/2014-CJFS
15. Harrison DE, Strong R, Reifsnyder P, et al. Astaxanthin and meclizine extend lifespan in UM-HET3 male mice; fisetin, SG1002 (hydrogen sulfide donor), dimethyl fumarate, mycophenolic acid, and 4-phenylbutyrate do not significantly affect lifespan in either sex at the doses and schedules used. GeroScience. 2023; 46(1): 795-816. doi: 10.1007/s11357-023-01011-0 DOI: https://doi.org/10.1007/s11357-023-01011-0
16. Müller L, Fröhlich K, Böhm V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chemistry. 2011; 129(1): 139-148. doi: 10.1016/j.foodchem.2011.04.045 DOI: https://doi.org/10.1016/j.foodchem.2011.04.045
17. Han H, Cui W, Wang L, et al. Lutein Prevents High Fat Diet‐Induced Atherosclerosis in ApoE‐Deficient Mice by Inhibiting NADPH Oxidase and Increasing PPAR Expression. Lipids. 2015; 50(3): 261-273. doi: 10.1007/s11745-015-3992-1 DOI: https://doi.org/10.1007/s11745-015-3992-1
18. Keegan G, Pardhan S, Chichger H. Lutein and zeaxanthin attenuates VEGF-induced neovascularisation in human retinal microvascular endothelial cells through a Nox4-dependent pathway. Experimental Eye Research. 2020; 197: 108104. doi: 10.1016/j.exer.2020.108104 DOI: https://doi.org/10.1016/j.exer.2020.108104
19. Fernández-Robredo P, Sádaba LM, Salinas-Alamán A, et al. Effect of Lutein and Antioxidant Supplementation on VEGF Expression, MMP-2 Activity, and Ultrastructural Alterations in Apolipoprotein E-Deficient Mouse. Oxidative Medicine and Cellular Longevity. 2013; 2013: 1-11. doi: 10.1155/2013/213505 DOI: https://doi.org/10.1155/2013/213505
20. Sharavana G, Baskaran V. Lutein downregulates retinal vascular endothelial growth factor possibly via hypoxia inducible factor 1 alpha and X-box binding protein 1 expression in streptozotocin induced diabetic rats. Journal of Functional Foods. 2017; 31: 97-103. doi: 10.1016/j.jff.2017.01.023 DOI: https://doi.org/10.1016/j.jff.2017.01.023
21. Leermakers ET, Darweesh SK, Baena CP, et al. The effects of lutein on cardiometabolic health across the life course: a systematic review and meta-analysis. The American Journal of Clinical Nutrition. 2016; 103(2): 481-494. doi: 10.3945/ajcn.115.120931 DOI: https://doi.org/10.3945/ajcn.115.120931
22. Hirahatake KM, Jacobs DR, Gross MD, et al. The Association of Serum Carotenoids, Tocopherols, and Ascorbic Acid With Rapid Kidney Function Decline: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Journal of Renal Nutrition. 2019; 29(1): 65-73. doi: 10.1053/j.jrn.2018.05.008 DOI: https://doi.org/10.1053/j.jrn.2018.05.008
23. Qiao YQ, Jiang PF, Gao YZ. Lutein prevents osteoarthritis through Nrf2 activation and downregulation of inflammation. Archives of Medical Science. 2018; 14(3): 617-624. doi: 10.5114/aoms.2016.59871 DOI: https://doi.org/10.5114/aoms.2016.59871
24. Hammond BR, Miller LS, Bello MO, et al. Effects of Lutein/Zeaxanthin Supplementation on the Cognitive Function of Community Dwelling Older Adults: A Randomized, Double-Masked, Placebo-Controlled Trial. Frontiers in Aging Neuroscience. 2017; 9. doi: 10.3389/fnagi.2017.00254 DOI: https://doi.org/10.3389/fnagi.2017.00254
25. Hoffman R, Sultan LD, Saada A, et al. Astaxanthin extends lifespan via altered biogenesis of the mitochondrial respiratory chain complex III. Available online: https://www.biorxiv.org/content/10.1101/698001v1 (accessed on 3 December 2024).
26. Nishida Y, Nawaz A, Hecht K, et al. Astaxanthin as a Novel Mitochondrial Regulator: A New Aspect of Carotenoids, beyond Antioxidants. Nutrients. 2021; 14(1): 107. doi: 10.3390/nu14010107 DOI: https://doi.org/10.3390/nu14010107
27. Nishida Y, Nawaz A, Kado T, et al. Astaxanthin stimulates mitochondrial biogenesis in insulin resistant muscle via activation of AMPK pathway. Journal of Cachexia, Sarcopenia and Muscle. 2020; 11(1): 241-258. doi: 10.1002/jcsm.12530 DOI: https://doi.org/10.1002/jcsm.12530
28. Wang Y, Chen X, Baker JS, et al. Astaxanthin promotes mitochondrial biogenesis and antioxidant capacity in chronic high-intensity interval training. European Journal of Nutrition. 2023; 62(3): 1453-1466. doi: 10.1007/s00394-023-03083-2 DOI: https://doi.org/10.1007/s00394-023-03083-2
29. Nair SVG, Ghanam K, Deshpande J, et al. Lutein and Zeaxanthin Isomers Induces Mitochondrial Biogenesis and Improves Endurance Capacity in Muscle Cells. EC Ophthalmology. 2018; 9(9): 658-668.
30. Xie K, Ngo S, Rong J, et al. Modulation of mitochondrial respiration underpins neuronal differentiation enhanced by lutein. Neural Regeneration Research. 2019; 14(1): 87. doi: 10.4103/1673-5374.243713 DOI: https://doi.org/10.4103/1673-5374.243713
31. Rubin LP, Chan GM, Barrett-Reis BM, et al. Effect of carotenoid supplementation on plasma carotenoids, inflammation and visual development in preterm infants. Journal of Perinatology. 2011; 32(6): 418-424. doi: 10.1038/jp.2011.87 DOI: https://doi.org/10.1038/jp.2011.87
32. Giampietri M, Lorenzoni F, Moscuzza F, et al. Lutein and Neurodevelopment in Preterm Infants. Frontiers in Neuroscience. 2016; 10. doi: 10.3389/fnins.2016.00411 DOI: https://doi.org/10.3389/fnins.2016.00411
33. Šimat V, Rathod N, Čagalj M, et al. Astaxanthin from Crustaceans and Their Byproducts: A Bioactive Metabolite Candidate for Therapeutic Application. Marine Drugs. 2022; 20(3): 206. doi: 10.3390/md20030206 DOI: https://doi.org/10.3390/md20030206
34. Torrissen OJ, Christiansen R. Requirements for carotenoids in fish diets. Journal of Applied Ichthyology. 1995; 11(3-4): 225-230. doi: 10.1111/j.1439-0426.1995.tb00022.x DOI: https://doi.org/10.1111/j.1439-0426.1995.tb00022.x
35. Keleştemur GT, Çoban OE. Effects of The β-Carotene on the Growth Performance and Skin Pigmentation of Rainbow Trout (Oncorhynchus mykiss, W. 1792). Journal of Fisheries & Livestock Production. 2016; 4: 164. doi: 10.4172/2332-2608.1000164 DOI: https://doi.org/10.4172/2332-2608.1000164
36. Perera CO, Yen GM. Functional Properties of Carotenoids in Human Health. International Journal of Food Properties. 2007; 10(2): 201-230. doi: 10.1080/10942910601045271 DOI: https://doi.org/10.1080/10942910601045271
37. Moretti VM, Mentasti T, Bellagamba F, et al. Determination of astaxanthin stereoisomers and colour attributes in flesh of rainbow trout (Oncorhynchus mykiss) as a tool to distinguish the dietary pigmentation source. Food Additives and Contaminants. 2006; 23(11): 1056-1063. doi: 10.1080/02652030600838399 DOI: https://doi.org/10.1080/02652030600838399
38. Brotosudarmo THP, Limantara L, Setiyono E, et al. Structures of Astaxanthin and Their Consequences for Therapeutic Application. International Journal of Food Science. 2020; 2020: 1-16. doi: 10.1155/2020/2156582 DOI: https://doi.org/10.1155/2020/2156582
39. Zajac G, Machalska E, Kaczor A, et al. Structure of supramolecular astaxanthin aggregates revealed by molecular dynamics and electronic circular dichroism spectroscopy. Physical Chemistry Chemical Physics. 2018; 20(26): 18038-18046. doi: 10.1039/c8cp01742e DOI: https://doi.org/10.1039/C8CP01742E
40. Dai M, Li C, Yang Z, et al. The Astaxanthin Aggregation Pattern Greatly Influences Its Antioxidant Activity: A Comparative Study in Caco-2 Cells. Antioxidants. 2020; 9(2): 126. doi: 10.3390/antiox9020126 DOI: https://doi.org/10.3390/antiox9020126
41. Todorović B, Grujić VJ, Krajnc AU, et al. Identification and Content of Astaxanthin and Its Esters from Microalgae Haematococcus pluvialis by HPLC-DAD and LC-QTOF-MS after Extraction with Various Solvents. Plants. 2021; 10(11): 2413. doi: 10.3390/plants10112413 DOI: https://doi.org/10.3390/plants10112413
42. Capelli B, Talbott S, Ding L. Astaxanthin sources: Suitability for human health and nutrition. Functional Foods in Health and Disease. 2019; 9(6): 430. doi: 10.31989/ffhd.v9i6.584 DOI: https://doi.org/10.31989/ffhd.v9i6.584
43. Capelli B, Bagchi D, Cysewski GR. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods. 2013; 12(4): 145-152. doi: 10.1007/s13749-013-0051-5 DOI: https://doi.org/10.1007/s13749-013-0051-5
44. Mariutti LRB, Mercadante AZ. Carotenoid esters analysis and occurrence: What do we know so far? Archives of Biochemistry and Biophysics. 2018; 648: 36-43. doi: 10.1016/j.abb.2018.04.005 DOI: https://doi.org/10.1016/j.abb.2018.04.005
45. Pott I, Breithaupt DE, Carle R. Detection of unusual carotenoid esters in fresh mango (Mangifera indica L. cv. ’Kent’). Phytochemistry. 2003; 64(4): 825–829. doi: 10.1016/s0031-9422(03)00466-7 DOI: https://doi.org/10.1016/S0031-9422(03)00466-7
46. Zhong L, Gustavsson KE, Oredsson S, et al. Determination of free and esterified carotenoid composition in rose hip fruit by HPLC-DAD-APCI+-MS. Food Chemistry. 2016; 210: 541-550. doi: 10.1016/j.foodchem.2016.05.002 DOI: https://doi.org/10.1016/j.foodchem.2016.05.002
47. Gregory GK, Chen T, Philip T. Quantitative Analysis of Lutein Esters in Marigold Flowers (Tagetes erecta) by High Performance Liquid Chromatography. Journal of Food Science. 1986; 51(4): 1093-1094. doi: 10.1111/j.1365-2621.1986.tb11248.x DOI: https://doi.org/10.1111/j.1365-2621.1986.tb11248.x
48. Olmedilla-Alonso B, Granado-Lorencio F, Castro-Feito J, et al. Bioavailability of Lutein from Marigold Flowers (Free vs. Ester Forms): A Randomised Cross-Over Study to Assess Serum Response and Visual Contrast Threshold in Adults. Nutrients. 2024; 16(10): 1415. doi: 10.3390/nu16101415 DOI: https://doi.org/10.3390/nu16101415
49. Bowen PE, Herbst-Espinosa SM, Hussain EA, et al. Esterification Does Not Impair Lutein Bioavailability in Humans. The Journal of Nutrition. 2002; 132(12): 3668-3673. doi: 10.1093/jn/132.12.3668 DOI: https://doi.org/10.1093/jn/132.12.3668
50. Requena-Ramírez MD, Rodríguez-Suárez C, Hornero-Méndez D, et al. Lutein esterification increases carotenoid retention in durum wheat grain. A step further in breeding and improving the commercial and nutritional quality during grain storage. Food Chemistry. 2024; 435: 137660. doi: 10.1016/j.foodchem.2023.137660 DOI: https://doi.org/10.1016/j.foodchem.2023.137660
51. Ouyang M, Huang Y, Wang Y, et al. Stability of carotenoids and carotenoid esters in pumpkin (Cucurbita maxima) slices during hot air drying. Food Chemistry. 2022; 367: 130710. doi: 10.1016/j.foodchem.2021.130710 DOI: https://doi.org/10.1016/j.foodchem.2021.130710
52. Aoi W, Maoka T, Abe R, et al. Comparison of the effect of non-esterified and esterified astaxanthins on endurance performance in mice. Journal of Clinical Biochemistry and Nutrition. 2018; 62(2): 161-166. doi: 10.3164/jcbn.17-89 DOI: https://doi.org/10.3164/jcbn.17-89
53. Ngoc NB, Lv P, Zhao WE. Suppressive effects of lycopene and β-carotene on the viability of the human esophageal squamous carcinoma cell line EC109. Oncology Letters. 2018; 15(5): 6727–6732. doi: 10.3892/ol.2018.8175
54. Zhang X, Zhao WE, Hu L, et al. Carotenoids inhibit proliferation and regulate expression of peroxisome proliferators-activated receptor gamma (PPARγ) in K562 cancer cells. Archives of Biochemistry and Biophysics. 2011; 512(1): 96-106. doi: 10.1016/j.abb.2011.05.004 DOI: https://doi.org/10.1016/j.abb.2011.05.004
55. Zhao WE, Shi G, Gu H, et al. Role of PPARγ in the nutritional and pharmacological actions of carotenoids. Research and Reports in Biochemistry. Published online April 2016: 13. doi: 10.2147/rrbc.s83258 DOI: https://doi.org/10.2147/RRBC.S83258
56. Lin JH, Lee DJ, Chang JS. Lutein production from biomass: Marigold flowers versus microalgae. Bioresource Technology. 2015; 184: 421-428. doi: 10.1016/j.biortech.2014.09.099 DOI: https://doi.org/10.1016/j.biortech.2014.09.099
57. Papapostolou H, Kachrimanidou V, Alexandri M, et al. Natural Carotenoids: Recent Advances on Separation from Microbial Biomass and Methods of Analysis. Antioxidants. 2023; 12(5): 1030. doi: 10.3390/antiox12051030 DOI: https://doi.org/10.3390/antiox12051030
58. Li F, Cai M, Lin M, et al. Enhanced Biomass and Astaxanthin Production of Haematococcus pluvialis by a Cell Transformation Strategy with Optimized Initial Biomass Density. Marine Drugs. 2020; 18(7): 341. doi: 10.3390/md18070341 DOI: https://doi.org/10.3390/md18070341
59. Ariyadasa TU, Thevarajah B, Anthonio RADP, et al. From present to prosperity: assessing the current status and envisioning opportunities in the industrial-scale cultivation of Haematococcus pluvialis for astaxanthin production. Phytochemistry Reviews. 2023; 23(3): 749-779. doi: 10.1007/s11101-023-09906-8 DOI: https://doi.org/10.1007/s11101-023-09906-8
60. An Y, Kim T, Byeon H, et al. Improved Production of Astaxanthin from Haematococcus pluvialis Using a Hybrid Open–Closed Cultivation System. Applied Sciences. 2024; 14(3): 1104. doi: 10.3390/app14031104 DOI: https://doi.org/10.3390/app14031104
61. McClure DD, Nightingale JK, Luiz A, et al. Pilot-scale production of lutein using Chlorella vulgaris. Algal Research. 2019; 44: 101707. doi: 10.1016/j.algal.2019.101707 DOI: https://doi.org/10.1016/j.algal.2019.101707
62. Dineshkumar R, Subramanian G, Dash SK, et al. Development of an optimal light-feeding strategy coupled with semi-continuous reactor operation for simultaneous improvement of microalgal photosynthetic efficiency, lutein production and CO2 sequestration. Biochemical Engineering Journal. 2016; 113: 47-56. doi: 10.1016/j.bej.2016.05.011 DOI: https://doi.org/10.1016/j.bej.2016.05.011
63. Patel AK, Vadrale AP, Singhania RR, et al. Enhanced mixotrophic production of lutein and lipid from potential microalgae isolate Chlorella sorokiniana C16. Bioresource Technology. 2023; 386: 129477. doi: 10.1016/j.biortech.2023.129477 DOI: https://doi.org/10.1016/j.biortech.2023.129477
64. Vadrale AP, Dong CD, Haldar D, et al. Bioprocess development to enhance biomass and lutein production from Chlorella sorokiniana Kh12. Bioresource Technology. 2023; 370: 128583. doi: 10.1016/j.biortech.2023.128583 DOI: https://doi.org/10.1016/j.biortech.2023.128583
65. Ma R, Zhang Z, Ho SH, et al. Two-stage bioprocess for hyper-production of lutein from microalga Chlorella sorokiniana FZU60: Effects of temperature, light intensity, and operation strategies. Algal Research. 2020; 52: 102119. doi: 10.1016/j.algal.2020.102119 DOI: https://doi.org/10.1016/j.algal.2020.102119
66. Xie Y, Li J, Ma R, et al. Bioprocess operation strategies with mixotrophy/photoinduction to enhance lutein production of microalga Chlorella sorokiniana FZU60. Bioresource Technology. 2019; 290: 121798. doi: 10.1016/j.biortech.2019.121798 DOI: https://doi.org/10.1016/j.biortech.2019.121798
67. Cordero BF, Obraztsova I, Couso I, et al. Enhancement of Lutein Production in Chlorella sorokiniana (Chorophyta) by Improvement of Culture Conditions and Random Mutagenesis. Marine Drugs. 2011; 9(9): 1607-1624. doi: 10.3390/md9091607 DOI: https://doi.org/10.3390/md9091607
68. Gabrielyan DA, Gabel BV, Sinetova MA, et al. Optimization of CO2 Supply for the Intensive Cultivation of Chlorella sorokiniana IPPAS C-1 in the Laboratory and Pilot-Scale Flat-Panel Photobioreactors. Life. 2022; 12(10): 1469. doi: 10.3390/life12101469 DOI: https://doi.org/10.3390/life12101469
69. Heo J, Shin DS, Cho K, et al. Indigenous microalga Parachlorella sp. JD-076 as a potential source for lutein production: Optimization of lutein productivity via regulation of light intensity and carbon source. Algal Research. 2018; 33: 1-7. doi: 10.1016/j.algal.2018.04.029 DOI: https://doi.org/10.1016/j.algal.2018.04.029
70. Mehariya S, Plöhn M, Leon-Vaz A, et al. Improving the content of high value compounds in Nordic Desmodesmus microalgal strains. Bioresource Technology. 2022; 359: 127445. doi: 10.1016/j.biortech.2022.127445 DOI: https://doi.org/10.1016/j.biortech.2022.127445
71. Kona R, Pallerla P, Addipilli R, et al. Lutein and β-carotene biosynthesis in Scenedesmus sp. SVMIICT1 through differential light intensities. Bioresource Technology. 2021; 341: 125814. doi: 10.1016/j.biortech.2021.125814 DOI: https://doi.org/10.1016/j.biortech.2021.125814
72. Tran HD, Do TT, Le TL, et al. Cultivation of Haematococcus pluvialis for astaxanthin production on angled bench-scale and large-scale biofilm-based photobioreactors. Vietnam Journal of Science, Technology and Engineering. 2019; 61(3): 61–70. doi: 10.31276/VJSTE.61(3).61-70 DOI: https://doi.org/10.31276/VJSTE.61(3).61-70




.jpg)
.jpg)

