Stability of antioxidative, peroxidative, and stress genes of food materials in different packaging materials under refrigeration
DOI:
https://doi.org/10.18686/fnc289Keywords:
beta-carotene; electrolytes; GSH; lipid peroxidation; packaging materials; stress genes; vitamin CAbstract
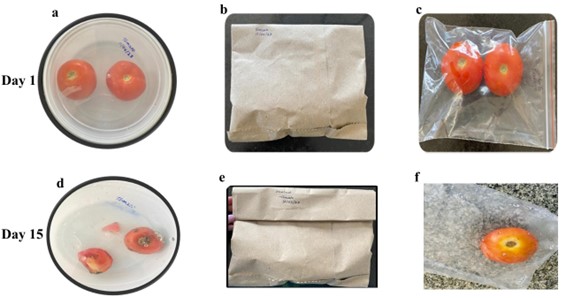
Traditional packaging materials for food include glass, metal, paper, paperboard, and plastic. The stability of food antioxidants depends on storage temperature, storage time, and type of packaging material. After stress exposure, gene expression is tightly controlled and reversible using various molecular pathways that are very dependent on specific stress and organism type. The current study examined the stability of lipid peroxidative, stress genes, and antioxidants in fruits and vegetables stored in various packaging materials under refrigeration. A plastic container, a brown paper bag, and a ziplock bag were utilized to store tomatoes and lemons under refrigeration (5–6 ℃), and the parameters were examined on Day 0 (control), Day 1, and Day 15. The values of the parameters were estimated via various technical methods, while the expressions of genes were determined using the process of polymerase chain reaction and visualized using agarose gel electrophoresis. The stability of antioxidants, lipid peroxidative, and electrolyte levels of the tomatoes and lemons stored in the various packaging materials were found to be significantly altered on Day 15 compared with those of the control. In addition, the expressions of stress genes of the tomatoes stored under refrigeration in the various packaging materials, especially the plastic container, were significantly downregulated on Day 15 compared with those of the control. In conclusion, no packaging materials are suitable for retaining the levels of antioxidants, lipid peroxidative, and stress genes of food materials under refrigeration.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Author(s)

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
1. Pandey S, Sharma K, Gundabala V. Antimicrobial bio-inspired active packaging materials for shelf life and safety development: A review. Food Bioscience. 2022; 48: 101730. doi: 10.1016/j.fbio.2022.101730
2. Korte I, Petry M, Kreyenschmidt J. Antimicrobial activity of different coatings for packaging materials containing functional extenders against selected microorganisms typical for food. Food Control. 2023; 148: 109669. doi: 10.1016/j.foodcont.2023.109669
3. Kaur N, Somasundram C, Razali Z, et al. Aloe vera/chitosan-based edible film with enhanced antioxidant, antimicrobial, thermal, and barrier properties for sustainable food preservation. Polymers. 2024; 16(2): 242. doi: 10.3390/polym16020242
4. Basavegowda N, Baek KH. Advances in functional biopolymer-based nanocomposites for active food packaging applications. Polymers. 2021; 13(23): 4198. doi: 10.3390/polym13234198
5. Hu J, Xu X, Song Y, et al. Microplastics in widely used polypropylene-made food containers. Toxics. 2022; 10(12): 762. doi: 10.3390/toxics10120762
6. Jo Y, Kim E, Kim S, et al. Delayed Quality Deterioration of Low-Moisture Cereal-Based Snack by Storing in an Active Filler-Embedded LDPE Zipper Bag. Foods. 2022; 11(12): 1704. doi: 10.3390/foods11121704
7. Meščić Macan A, Gazivoda Kraljević T, Raić-Malić S. Therapeutic perspective of vitamin C and its derivatives. Antioxidants. 2019; 8(8): 247. doi: 10.3390/antiox8080247
8. Unsal V, Dalkıran T, Çiçek M, Kölükçü E. The role of natural antioxidants against reactive oxygen species produced by cadmium toxicity: a review. Advanced pharmaceutical bulletin. 2020; 10(2): 184. doi: 10.34172/apb.2020.023
9. Kaźmierczak-Barańska J, Boguszewska K, Adamus-Grabicka A, et al. Two faces of vitamin C—Antioxidative and pro-oxidative agent. Nutrients. 2020; 12(5): 1501. doi: 10.3390/nu12051501
10. Kadzińska J, Janowicz M, Kalisz S, et al. An overview of fruit and vegetable edible packaging materials. Packaging Technology and Science. 2019; 32(10): 483–495. doi: 10.1002/pts.2440.
11. Liang Z, Veronica V, Huang J, et al. Combined effects of plant food processing by-products and high oxygen modified atmosphere packaging on the storage stability of beef patties. Food Control. 2022; 133: 108586. doi: 10.1016/j.foodcont.2021.108586
12. Balaswamy K, Prabhakara Rao PG, Sulochanamma G, et al. Stability of β-Carotene in Pumpkin Flour Fortified Vermicelli. The Indian Journal of Nutrition and Dietetics. 2022; 59(3): 310–322. doi: 10.21048/IJND.2022.59.3.28959
13. Christodoulou MC, Orellana Palacios JC, Hesami G, et al. Spectrophotometric methods for measurement of antioxidant activity in food and pharmaceuticals. Antioxidants. 2022; 11(11): 2213. doi: 10.3390/antiox11112213
14. Al-Temimi AA, Al-Mossawi AE, Al-Hilifi SA, et al. Glutathione for food and health applications with emphasis on extraction, identification, and quantification methods: A review. Metabolites. 2023; 13(4): 465. doi: 10.3390/metabo13040465
15. Wen B, Zhang F, Wu X, et al. Characterization of the tomato (Solanum lycopersicum) pectin methylesterases: Evolution, activity of isoforms and expression during fruit ripening. Frontiers in Plant Science. 2020; 11: 238. doi: 10.3389/fpls.2020.00238
16. Biswas AK, Sahoo J, Chatli MK. A simple UV-Vis spectrophotometric method for determination of β-carotene content in raw carrot, sweet potato and supplemented chicken meat nuggets. LWT-Food Science and Technology. 2011; 44(8): 1809–1813. doi: 10.1016/j.lwt.2011.03.017
17. Cohn VH, Lyle J. A fluorometric assay for glutathione. Analytical Biochemistry. 1966; 14(3): 434–440. doi: 10.1016/0003-2697(66)90286-7
18. Tipple TE, Rogers LK. Methods for the determination of plasma or tissue glutathione levels. Developmental Toxicology: Methods and Protocols. 2012; 889: 315–324. doi: 10.1007/978-1-61779-867-2_20
19. Zeb A, Ullah F. A simple spectrophotometric method for the determination of thiobarbituric acid reactive substances in fried fast foods. Journal of Analytical Methods in Chemistry. 2016; 2016(1): 9412767. doi: 10.1155/2016/9412767
20. Worth HG. A comparison of the measurement of sodium and potassium by flame photometry and ion-selective electrode. Annals of clinical biochemistry. 1985; 22(4): 343–350. doi: 10.1177/000456328502200402
21. Marsh K, Bugusu B. Food packaging—Roles, materials, and environmental issues. Journal of Food Science. 2007; 72(3): R39–R55. doi: 10.1111/j.1750-3841.2007.00301.x
22. Griffin RC, Sacharow S, Brody AL, et al. Origins of Packaging Development in Principles of Package Development. Springer; 1985. pp. 1–14.
23. Oyetade OA, Oyeleke GO, Adegoke BM, et al. Stability studies on ascorbic acid (vitamin C) from different sources. Journal of Applied Chemistry. 2012; 2(4): 20–24.
24. Li L, Yang Y, Xu Q, et al. The Or gene enhances carotenoid accumulation and stability during post-harvest storage of potato tubers. Molecular plant. 2012; 5(2): 339–352. doi: 10.1093/mp/ssr099
25. Alamri MS, Qasem AA, Mohamed AA, et al. Food packaging’s materials: A food safety perspective. Saudi Journal of Biological Sciences. 2021; 28(8): 4490–4499. doi: 10.1016/j.sjbs.2021.04.047
26. Akhavan O, Ghaderi E, Akhavan A. Size-dependent genotoxicity of graphene nanoplatelets in human stem cells. Biomaterials. 2012; 33(32): 8017–8025. doi: 10.1016/j.biomaterials.2012.07.040
27. Hashemi E, Akhavan O, Shamsara M, et al. Synthesis and cyto-genotoxicity evaluation of graphene on mice spermatogonial stem cells. Colloids and Surfaces B: Biointerfaces. 2016; 146: 770–776. doi: 10.1016/j.colsurfb.2016.07.019
28. Lin Z, Long F, Kang R, et al. The lipid basis of cell death and autophagy. Autophagy. 2024; 20(3): 469–488. doi: 10.1080/15548627.2023.2259732.
29. Zakrys PI, O’Sullivan MG, Allen P, et al. Consumer acceptability and physiochemical characteristics of modified atmosphere packed beef steaks. Meat science. 2009; 81(4): 720–725. doi: 10.1016/j.meatsci.2008.10.024
30. Xiong YL. Antioxidants in muscle foods: Nutritional strategies to improve quality. In: Decker E, Faustman C, Lopez-Bote CJ (editors). Protein oxidation and implications for muscle food quality. John Wiley and Sons; 2000. pp. 85–111.
31. Stedwell RE, Allen KM, Binder LS. Hypokalemic paralyses: A review of the etiologies, pathophysiology, presentation, and therapy. The American journal of emergency medicine. 1992; 10(2): 143–148. doi: 10.1016/0735-6757(92)90048-3
32. Viera AJ, Wouk N. Potassium disorders: Hypokalemia and hyperkalemia. American family physician. 2015; 92(6): 487–495.
33. Janjarasskul T, Krochta JM. Edible packaging materials. Annual review of food science and technology. 2010; 1(1): 415–448. doi: 10.1146/annurev.food.080708.100836
34. Wu HC, Bulgakov VP, Jinn TL. Pectin methylesterases: Cell wall remodeling proteins are required for plant response to heat stress. Frontiers in plant science. 2018; 9: 1612. doi: 10.3389/fpls.2018.01612
35. Downie A, Miyazaki S, Bohnert H, et al. Expression profiling of the response of Arabidopsis thaliana to methanol stimulation. Phytochemistry. 2004, 65(16): 2305–2316. doi: 10.1016/j.phytochem.2004.07.006
36. Folsom JJ, Begcy K, Hao X, et al. Rice fertilization-independent endosperm1 regulates seed size under heat stress by controlling early endosperm development. Plant physiology. 2014; 165(1): 238–248. doi: 10.1104/pp.113.232413
37. Fragkostefanakis S, Mesihovic A, Simm S, et al. HsfA2 controls the activity of developmentally and stress-regulated heat stress protection mechanisms in tomato male reproductive tissues. Plant physiology. 2016; 170(4): 2461–2477. doi: 10.1104/pp.15.01913
38. Kashyap D, Sharma A, Tuli HS, et al. Apigenin: A natural bioactive flavone-type molecule with promising therapeutic function. Journal of Functional Foods. 2018; 48: 457–471. doi: 10.1016/j.jff.2018.07.037
39. Madunić J, Madunić IV, Gajski G, et al. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer letters. 2018; 413: 11–22.
40. Pamunuwa G, Karunaratne DN, Waisundara VY. Antidiabetic properties, bioactive constituents, and other therapeutic effects of Scoparia dulcis. Evidence‐Based Complementary and Alternative Medicine. 2016; 2016(1): 8243215. doi: 10.1155/2016/8243215
41. Feszterová M, Kowalska M, Mišiaková M. Stability of Vitamin C content in plant and vegetable juices under different storing conditions. Applied Sciences. 2023; 13(19): 10640. doi: 10.3390/app131910640
42. Roy J, Islam MN, Yasmin S, Mahomud MS. Improvement of quality and shelf-life of tomatoes with Aloe vera coatings enriched with tulsi extract. Applied Food Research. 2024; 4(2):100449. doi: 10.1016/j.afres.2024.100449
43. Wu Y, Zhang S, Yang H, et al. Methyl jasmonate and salicylic acid treatment changes the nutritional quality, antioxidant profile and gene expression of postharvest blackberry fruit. Postharvest Biology and Technology. 2025; 219:113205. doi: 10.1016/j.postharvbio.2024.113205
44. Kayshar MS, Rana J, Arifin MS, et al. Natural Alternatives in Sports Nutrition: Formulation and Quality Evaluation of an Isotonic Sports Drink Using Dates of Ajwa Variety (Phoenix dactylifera L.). Applied Food Research. 2024; 4(2): 100618. doi: 10.1016/j.afres.2024.100618
45. Feng Y, Huang L, Zeng Y, et al. Cloning, Expression and Enzymatic Characterization of Pectin Methyl Esterase from Populus trichocarpa and Its Application. Processes. 2024; 12(7): 1511. doi: 10.3390/ pr12071511
46. Gao S, Peng J, Rong M, et al. Screening and validation of reference genes in Dracaena cochinchinensis using quantitative real-time PCR. Scientific Reports. 2024; 14(1): 6165. doi: 10.1038/s41598-024-52754-5




.jpg)
.jpg)

