Postbiotics: Potential as functional ingredients—A review
DOI:
https://doi.org/10.18686/fnc241Keywords:
postbiotics; functional foods; health benefits; probiotic viability; inactivated probiotic cells; cell fractionsAbstract
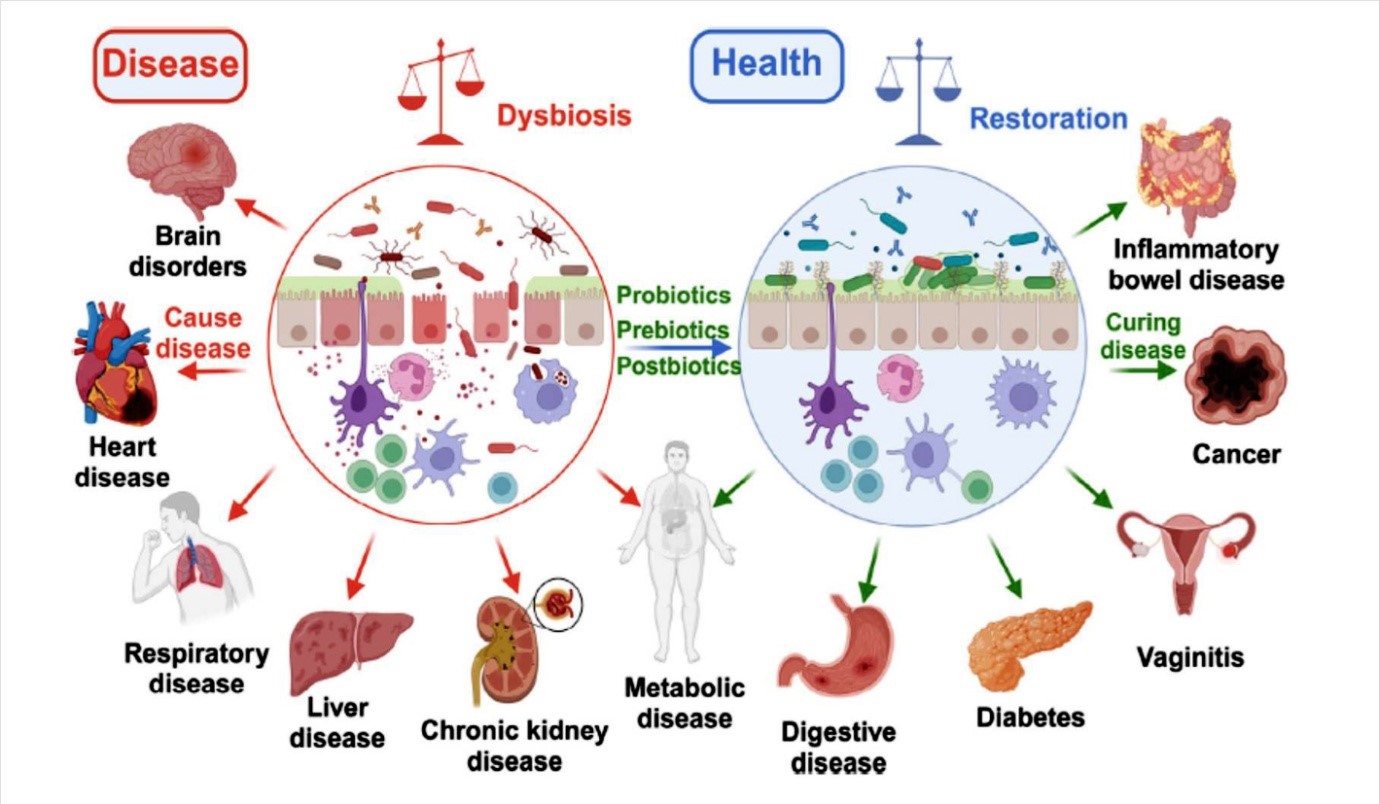
A review of the importance of probiotic viability, the functions of viable and postbiotic microorganisms, and their use in nutritious foods is presented. Reviews and studies on the effectiveness of dead, inactivated, or destroyed probiotic cells for health benefits were taken into consideration. Among the keywords used in the data search were “probiotic viability”, “postbiotics”, “viable or killed”, “inactivated probiotic cells”, and “functional foods”. Google, Pub Med, ResearchGate, and others were the search platforms used. Probiotics are beneficial to health, but they have certain drawbacks. In this review of the literature and current research, even dead cells have been shown to have positive effects on health. The purpose of this work is to demonstrate that, in addition to live probiotics, deactivated or non-living probiotic cells can also effectively extend health benefits. Numerous postbiotic substances derived from a wide variety of microorganisms can enhance gut health and extend health advantages. Not all live probiotic cultures are equally effective, and as a result, inactive or dead cells do not possess similar functional properties to provide health benefits for all diseases. Postbiotics can be classified into three groups based on research findings regarding their functional properties: (i) postbiotics that are less effective than probiotics, (ii) postbiotics that are equally effective as probiotics, and (iii) postbiotics that are more effective than probiotics. When compared with live probiotics, the advantages of inactivated bacteria and/or purified compounds include safety, physiological effects, and pharmaceutical properties. Food manufacturers may be drawn to the effectiveness of non-viable probiotics or the cell fractions of probiotics for health benefits because these have some advantages over live probiotics, including longer shelf life, ease of transportation, and a lower need for refrigerated storage. The inclusion of non-viable probiotics or their cell fractions may have the potential of developing probiotic food formulation.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Surajit Sarkar

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
1. Yoha KS, Nida S, Dutta S, et al. Targeted delivery of probiotics: Perspectives on research and commercialization. Probiotics and Antimicrobial Proteins. 2021; 14: 15–48.
2. Essa MM, Bishir M, Bhat A, et al. Functional foods and their impact on health. Journal Food Science and Technology. 2021; 60: 820–834.
3. Baker MT, Lu P, Parrella JA, Leggette HR. Consumer acceptance toward functional foods: A scoping review. International Journal of Environmental Research and Public Health. 2022; 19: 1217.
4. Temple NJ. A rational definition for functional foods: A perspective. Frontiers in Nutrition. 2022; 9: 957516.
5. Bigliardi B, Galati F. Innovation trends in the food industry: The case of functional foods. Trends in Food Science & Technology. 2013; 31: 118–129.
6. Sarkar S. Potentiality of probiotic fruit yoghurt as a functional food—a review. Nutrition & Food Science. 2019; 2: 9.
7. Lenssen K, Bast A, de Boer A. Should botanical health claims be substantiated with evidence on traditional use? Reviewing the stakeholders’ arguments. PharmaNutrition. 2020; 14: 100232.
8. Hill C, Guarner F, Reid G, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology. 2014; 11: 506–514.
9. Sarkar S. Probiotics as functional foods: Documented health benefits. Nutrition & Food Science. 2013a; 43: 107–115.
10. Sarkar S. Potential of probiotics as pharmaceutical agent—a review. British Food Journal. 2013b; 115: 1658–1687.
11. Stavropoulou E, Bezirtzoglou E. Probiotics in medicine: A long debate. Frontiers in Immunology. 2020; 11: 2192.
12. Mishra S, Acharya S. A brief overview on probiotics: The health friendly microbes. Biomedical & Pharmacology Journal. 2021; 14: 1869–1880.
13. Das TK, Pradhan S, Chakrabarti S, et al. Current status of probiotic and related health benefits. Applied Food Research. 2022; 2: 100185.
14. de Almada CN, Almada CN, Martinez RCR, Sant’Ana AS. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends in Food Science & Technology. 2016; 58: 96–114.
15. Nataraj BH, Ali SA, Behare PV, Yadav H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microbial Cell Factories. 2020; 19: 168.
16. Cicenia A, Scirocco A, Carabotti M, et al. Postbiotic activities of Lactobacilli-derived factors. Journal of Clinical Gastroenterology. 2014; 48: 18–22.
17. Kim KW, Kang SS, Woo SJ, et al. Lipoteichoic acid of probiotic Lactobacillus plantarum attenuates Poly I: C-Induced IL-8 production in porcine intestinal epithelial cells. Frontiers in Microbiology. 2017; 8: 1827.
18. Liu Z, Zhang Z, Qiu L, et al. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. Journal Dairy Science. 2017; 100: 6895–6905.
19. Aguilar-Toala JE, Garcia-Varela R, Garcia HS, et al. Postbiotics: An evolving term within the functional foods field. Trends in Food Science & Technology. 2018; 75: 105–114.
20. Tsilingiri K, Rescigno M. Postbiotics: what else? Beneficial Microbes. 2013; 4: 101–107.
21. Ishikawa H, Kutsukake E, Fukui T, et al. Oral administration of heat-killed Lactobacillus plantarum strain b240 protected mice against Salmonella enterica Serovar typhimurium. Bioscience, Biotechnology, and Biochemistry. 2010; 74: 1338–1342.
22. Ou CC, Lin SL, Tsai JJ, Lin MY. Heat-killed lactic acid bacteria enhance immunomodulatory potential by skewing the immune response toward Th1 polarization. Journal of Food Science. 2011; 76: 260–267.
23. Taverniti V, Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes & Nutrition. 2011; 6: 261–274.
24. Neffe-Skocińska K, Rzepkowska A, Szydłowska A, Kołożyn-Krajewska D. Trends and possibilities of the use of probiotics in food production. Academic Press; 2018. pp. 65–94.
25. Calinoiu LF, Vodnar D, Precup GA. A Review: The probiotic bacteria viability under different conditions. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Food Science and Technology. 2016; 73, 55–60.
26. Karimi R, Mortazavian AM, Da Cruz AG. Viability of probiotic microorganisms in cheese during production and storage: A review. Dairy Science and Technology. 2011; 91: 283–308.
27. Minelli EB, Benini A. Relationship between number of bacteria and their probiotic effects. Microbial Ecology in Health and Disease. 2009; 20: 180–183.
28. Szajewska H, Skorka A, Ruszczynski M, Gieruszczak-Bialek D. Meta-analysis: Lactobacillus GG for treating acute gastroenteritis in children–updated analysis of randomised controlled trials. Alimentary Pharmacology & Therapeutics. 2013; 38: 467–476.
29. Caffarelli C, Cardinale F, Povesi-Dascola C, et al. Use of probiotics in pediatric infectious diseases. Expert Review of Anti-infective Therapy. 2015; 13: 1517–1535.
30. Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014; 64: 897–903.
31. Vitetta L, Coulson S, Thomsen M, et al. Probiotics, D-Lactic acidosis, oxidative stress and strain specificity. Gut Microbes. 2017; 8: 311–322.
32. Sniffen JC, McFarland LV, Evans CT, Goldstein EJC. Choosing an appropriate probiotic product for your patient: An evidence-based practical guide. PLoS One. 2018; 13: e0209205.
33. Varsha KK, Maheshwari AP, Nampoothiri KM. Accomplishment of probiotics in human health pertaining to immune-regulation and disease control. Clinical Nutrition ESPEN. 2021; 44: 26–37.
34. De Simone C. The unregulated probiotic market. Clinical Gastroenterology and Hepatology. 2019; 17: 809–817.
35. Shripada R, Gayatri AJ, Sanjay P. Paraprobiotics. Academic Press; 2020. pp. 39–49.
36. Kim M, Nam D, Kim S, et al. Enhancement of viability, acid, and bile tolerance and accelerated stability in lyophilized Weissella cibaria JW15 with protective agents. Food Science & Nutrition. 2018; 6: 1904–1913.
37. Akalın AS, Kesenkas H, Dinkci N, et al. Enrichment of probiotic ice cream with different dietary fibers: Structural characteristics and culture viability. Journal of Dairy Science. 2018; 101: 37–46.
38. Pique N, Berlanga M, Miñana-Galbis D. Health benefits of heat-killed (tyndallized) probiotics: An overview. International Journal of Molecular Science. 2019; 20: 2534.
39. Broeckx G, Vandenheuvel D, Claes IJJ, et al. Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. International Journal of Pharmaceutics. 2016; 505: 303–318.
40. Fenster K, Freeburg B, Hollard C, et al. The production and delivery of probiotics: A review of a practical approach. Microorganisms. 2019; 7: 83.
41. Fiore W, Arioli S, Guglielmetti S. The neglected microbial components of commercial probiotic formulations. Microorganisms. 2020; 8: 1177.
42. Fredua-Agyeman M, Gaisford S. Comparative survival of commercial probiotic formulations: Tests in biorelevant gastric fluids and real-time measurements using microcalorimetry. Beneficial Microbes. 2015; 6: 141–151.
43. Derrien M, van Hylckama, Vlieg JE. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends in Microbiology. 2015; 23: 354–366.
44. Sabikhi L, Babu R, Thompkinson DK, Kapila S. Resistance of microencapsulated Lactobacillus acidophilus LA1 to processing treatments and simulated gut conditions. Food Bioprocess Technology. 2010; 3: 586–593.
45. Brinques GB, Ayub MAZ. Effect of microencapsulation on survival of Lactobacillus plantarum in simulated gastrointestinal conditions, refrigeration, and yogurt. Journal of Food Engineering. 2021; 103: 123–128.
46. Segers ME, Lebeer S. Towards a better understanding of Lactobacillus rhamnosus GG–host interactions. Microbial Cell Factories. 2014; 13: 1–7.
47. Li F, Cheng CC, Zheng J, et al. Limosilactobacillus balticus sp. nov., Limosilactobacillus agrestis sp. nov., Limosilactobacillus albertensis sp. nov., Limosilactobacillus rudii sp. nov. and Limosilactobacillus fastidiosus sp. nov., five novel Limosilactobacillus species isolated from the vertebrate gastrointestinal tract, and proposal of six subspecies of Limosilactobacillus reuteri adapted to the gastrointestinal tract of specific vertebrate hosts. International Journal of Systematic and Evolutionary Microbiology. 2021; 71: 004644.
48. Ohishi A, Takahashi S, Ito Y, et al. Bifidobacterium septicemia associated with postoperative probiotic therapy in a neonate with omphalocele. Journal of Pediatrics. 2010; 156: 679–681.
49. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Systematic Review. 2017; 12: CD006095.
50. Deshpande G, Athalye-Jape G, Patole S. Para-probiotics for preterm neonates—The next frontier. Nutrition. 2018; 10: 871.
51. Sanders ME, Merenstein DJ, Ouwehand AC, et al. Probiotic use in at-risk populations. Journal of the American Pharmacists Association. 2016; 56: 680–686.
52. Crow JR, Davis SL, Chaykosky DM, et al. Probiotics and fecal microbiota transplant for primary and secondary prevention of Clostridium difficile infection. Pharmacotherapy. 2015; 35: 1016–1025.
53. Pamer EG. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science. 2016; 352: 535–538.
54. Draper K, Ley C, Parsonnet J. Probiotic guidelines and physician practice: A cross-sectional survey and overview of the literature. Beneficial Microbes. 2017; 8: 507–519.
55. de Marco S, Sichetti M, Muradyan D, et al. Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with LPS. Evidence Based Complementary Alternative of Medicine. 2018; 2018: 1756308.
56. Mazzantini D, Calvigioni M, Celandroni F, et al. Spotlight on the compositional quality of probiotic formulations marketed worldwide. Frontiers in Microbiology. 2021; 12: 693973.
57. Scott E, De Paepe K, Van de Wiele T. Postbiotics and their health modulatory biomolecules. Biomolecules. 2022; 12: 1640.
58. Salminen S, Collado MC, Endo A, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nature Reviews Gastroenterology & Hepatology. 2021; 18: 649–667.
59. Patani A, Balram D, Yadav VK, et al. Harnessing the power of nutritional antioxidants against adrenal hormone imbalance-associated oxidative stress. Front Endocrinology. 2023; 14: 1271521.
60. Rad AH, Kafil HA, Zavoshti HF, et al. Therapeutically effects of functional postbiotic foods. Clinical Excellence. 2022b; 10: 33–52.
61. Rad AH, Maleki LA, Kafil HS, et al. Postbiotics as novel health-promoting ingredients in functional foods. Health Promotion Perspectives. 2020a; 10: 3–4.
62. Gurunathan S, Thangaraj P, Das J, Kim JH. Postbiotics: Functional food materials and therapeutic agents for cancer, diabetes, and inflammatory diseases. Foods. 2024; 13: 89.
63. Szydlowska A, Sionek B. Probiotics and postbiotics as the functional food components affecting the immune response. Microorganisms. 2023; 11: 104.
64. Thorakkattu P, Khanashyam AC, Shah K, et al. Postbiotics: Current trends in food and pharmaceutical industry. Foods. 2022; 11: 3094.
65. Hernandez-Granados MJ, Franco-Robles E. Postbiotics in human health: Possible new functional ingredients? Food Research International. 2020; 137: 109660.
66. Sabahi S, Rad AH, Aghebati-Maleki L, et al. Postbiotics as the new frontier in food and pharmaceutical research. Critical Review in Food Science and Nutrition. 2023; 63: 8375–8402.
67. Nakamura S, Kuda T, An C, et al. Inhibitory effects of Leuconostoc mesenteroides 1RM3 isolated from narezushi, a fermented fish with rice, on Listeria monocytogenes infection to Caco-2 cells and A/J mice. Anaerobe. 2012; 18: 19–24.
68. Ramkrishna S, Chellamboli C, Anupama D. A revolution by probiotic and paraprobiotic in food industry: Review. Research Review International Journal Multidisciplinary. 2019; 4: 72–81.
69. Kim H, Kim H, Bang J, et al. Reduction of Bacillus cereus spores in sikhye, a traditional Korean rice beverage, by modified tyndallization processes with and without carbon dioxide injection. Letters in Applied Microbiology. 2012; 55: 218–223.
70. Daelemans S, Peeters L, Hauser B, Vandenplas Y. Recent advances in understanding and managing infantile colic. F1000 Research. 2018; 7: F1000.
71. Chen CY, Tsen HY, Lin CL, et al. Enhancement of the immune response against Salmonella infection of mice by heat-killed multispecies combinations of lactic acid bacteria. Journal of Medical Microbiology. 2013; 62:1657-1664.
72. Shin HS, Park SY, Lee DK, et al. Hypocholesterolemic effect of sonication-killed Bifidobacterium longum isolated from healthy adult Koreans in high cholesterol fed rats. Archives of Pharmacal Research. 2010; 33: 1425–1431.
73. Roman L, Padilla D, Acosta F, et al. The effect of probiotic Enterococcus gallinarum L-1 on the innate immune parameters of outstanding species to marine aquaculture. Journal of Applied Animal Research. 2015; 43: 177–183.
74. Habil N, Abate W, Beal J, Foey AD. Heat-killed probiotic bacteria differentially regulate colonic epithelial cell production of human β-defensin-2: Dependence on inflammatory cytokines. Beneficial Microbes. 2014; 5: 483–495.
75. Ditu LM, Chifiriuc MC, Bezirtzoglou E, et al. Immunomodulatory effect of non-viable components of probiotic culture stimulated with heat-inactivated Escherichia coli and Bacillus cereus on holoxenic mice. Microbial Ecology in Health and Disease. 2014; 25: 23239.
76. Sang LX, Chang B, Wang BY, et al. Live and heat-killed probiotic: Effects on chronic experimental colitis induced by dextran sulfate sodium (DSS) in rats. International Journal of Clinical Experimental Medicine. 2015; 8: 20072–20078.
77. Thakur BK, Saha P, Banik G, et al. Live and heat-killed probiotic Lactobacillus casei Lbs2 protects from experimental colitis through toll-like receptor 2-dependent induction of T-regulatory response. International Immunopharmacology. 2016; 36: 39–50.
78. Sandeep P, Kumari PS. Heat killed probiotic Lactobacillus plantarum induces NF kappa B factor compared with live bacteria and lipopolysaccharides on HEK-293 cell lines. International Journal of Pharma & Biosciences. 2016; 7: 438–441.
79. Lee MJ, Zang ZL, Choi EY, et al. Cytoskeleton reorganization and cytokine production of macrophages by bifidobacterial cells and cell-free extracts. Journal of Microbiology and Biotechnology. 2002; 12: 398–405.
80. Jorjao AL, de Oliveira FE, Leao MV, et al. Live and Heat-Killed Lactobacillus rhamnosus ATCC 7469 may induce modulatory cytokines profiles on macrophages RAW 264.7. Scientific World Journal. 2015; 2015: 716749.
81. Choi S, Kim Y, Han K, et al. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Letters in Applied Microbiology. 2006; 42: 452–458.
82. Lee JY, Nguyen DT, Park YS, et al. Organic acid profiling analysis in culture media of lactic acid bacteria by gas chromatography-mass spectrometry. Mass Spectrometry Letters. 2012; 3: 74–77.
83. Amaretti A, di Nunzio M, Pompei A, et al. Antioxidant properties of potentially probiotic bacteria: In vitro and in vivo activities. Applied Microbiology and Biotechnology. 2013; 97: 809–817.
84. Tiptiri-Kourpeti A, Spyridopoulou K, Santarmaki V, et al. Lactobacillus casei exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of TRAIL in colon carcinoma cells. PLoS One. 2016; 11: e0147960.
85. Nakamura F, Ishida Y, Sawada D, et al. Fragmented lactic acid bacterial cells activate peroxisome proliferator-activated receptors and ameliorate dyslipidemia in obese mice. Journal of Agricultural and Food Chemistry. 2016; 64: 2549–2559.
86. Dinic M, Lukic J, Djokic J, et al. Lactobacillus fermentum postbiotic-induced autophagy as potential approach for treatment of acetaminophen hepatotoxicity. Frontiers in Microbiology. 2017; 8: 594.
87. Aguilar-Toala JE, Hall FG, Urbizo-Reyes UC, et al. In silico prediction and in vitro assessment of multifunctional properties of postbiotics obtained from two probiotic bacteria. Probiotics and Antimicrobial Proteins. 2020; 12: 608–622.
88. Fiocco D, Longo A, Arena MP, et al. How probiotics face food stress: They get by with a little help. Critical Review in Food Science and Nutrition. 2020; 60: 1552–1580.
89. Barros CP, Guimaraes JTA, Esmerino E, et al. Paraprobiotics and postbiotics: Concepts and potential applications in dairy products. Current Opinion in Food Science. 2020; 32: 1–8.
90. Vinderola G, Sanders ME, Salminen S. The concept of postbiotics. Foods. 2022a; 11: 1077.
91. Adams CA. The probiotic paradox: Live and dead cells are biological response modifiers. Nutrition Research Reviews. 2010; 23: 37–46.
92. Ji J, Jin W, Liu SJ, et al. Probiotics, prebiotics, and postbiotics in health and disease. MedComm. 2023; 4: e420.
93. Venema K. Foreword. Beneficial Microbes. 2013; 4: 1–2.
94. Vallejo-Cordoba B, Castro-Lopez C, García HS, et al. Postbiotics and paraprobiotics: A review of current evidence and emerging trends. Advances in Food & Nutrition Research. 2020; 94: 1–34.
95. Teame T, Wang A, Xie M, et al. Paraprobiotics and postbiotics of probiotic Lactobacilli, their positive effects on the host and action mechanisms: A review. Frontiers in Nutrition. 2020; 7: 570344.
96. Warda AK, Rea K, Fitzgerald P, et al. Heat-killed lactobacilli alter both microbiota composition and behaviour. Behavioural Brain Research. 2019; 362: 213–223.
97. Majhenic AC, Lorbeg PM, Treven P. Enumeration and identification of mixed probiotic and lactic acid bacteria starter cultures. Wiley-Blackwell Press; 2017. pp. 207–251.
98. Guimaraes JT, Balthazar CF, Silva R, et al. Impact of probiotics and prebiotics on food texture. Current Opinion in Food Science. 2020; 33: 3844.
99. Zawistowska-Rojek A, Tyski S. Are probiotic really safe for humans? Poland Journal of Microbiology. 2018; 67: 251–258.
100. Siciliano RA, Reale A, Mazzeo MF, et al. Paraprobiotics: A new perspective for functional foods and nutraceuticals. Nutrients. 2021; 13: 1225.
101. Akter S, Park JH, Jung HK. Potential health-promoting benefits of paraprobiotics, inactivated probiotic cells. Journal of Microbiol Biotechnology. 2020; 30: 477–481.
102. Pujato SA, del L Quiberoni A, Candioti MC, et al. Leuconostoc citreum MB1 as biocontrol agent of Listeria monocytogenes in milk. Journal of Dairy Research. 2014; 81: 137–145.
103. Dong H, Rowland I, Thomas LV, Yaqoob P. Immunomodulatory effects of a probiotic drink containing Lactobacillus casei Shirota in healthy older volunteers. European Journal of Nutrition. 2013; 52: 1853–1863.
104. de Almada CN, De Almada CN, Martinez RCR, de Souza Sant’Ana A. Characterization of the intestinal microbiota and its interaction with probiotics and health impacts. Applied Microbiology and Biotechnology. 2015; 99: 4175–4199.
105. Scarpellini E, Basilico M, Rinninella E, et al. Probiotics and gut health. Minerva Gastroenterology (Torino). 2021; 67: 314–325.
106. Sartor RB. Gut microbiota: Diet promotes dysbiosis and colitis in susceptible hosts. Nature Reviews Gastroenterology & Hepatology. 2012; 9: 561–562.
107. Tlaskalova-Hogenova H, Stepankova R, Kozakova H, et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: Contribution of germ-free and gnotobiotic animal models of human diseases. Cellular & Molecular Immunology. 2011; 8: 110–120.
108. Wang T, Roest DIM, Smidt H, Zoetendal EG. We are what we eat: How diet impacts the gut microbiota in adulthood. Springer International Publishing; 2019b. pp. 259–283.
109. Ercolini D, Fogliano V. Food design to feed the human gut microbiota. Journal of Agricultural and Food Chemistry. 2018; 66: 3754–3758.
110. Sommer F, Backhed F. The gut microbiota—Masters of host development and physiology. Nature Reviews Microbiology. 2013; 11: 227–238.
111. Kamada N, Nunez G. Role of the gut microbiota in the development and function of lymphoid cells. Journal of Immunology. 2013; 190: 1389–1395.
112. Brown EM, Sadarangani M, Finlay BB. The role of the immune system in governing host-microbe interactions in the intestine. Nature Immunology. 2013; 14: 660–667.
113. Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nature Reviews Immunology. 2013; 13: 790–801.
114. Hills Jr RD, Pontefract BA, Mishcon HR, et al. Gut microbiome: Profound implications for diet and disease. Nutrients. 2019; 11: 1613.
115. Wang G, Huang S, Wang Y, et al. Bridging intestinal immunity and gut microbiota by metabolites. Cellular and Molecular Life Sciences. 2019a; 76: 3917–3937.
116. Natividad JMM, Verdu EF. Modulation of intestinal barrier by intestinal microbiota: Pathological and therapeutic implications. Pharmacological Research. 2013; 69: 42–51.
117. Den Besten G, van Eunen K, Groen AK, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research. 2013; 54: 2325–2340.
118. Baumler AJ, Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 2016; 535: 85–93.
119. Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016; 352: 539–544.
120. Ozma MA, Abbasi A, Akrami S, et al. Postbiotics as the key mediators of the gut microbiota-host interactions. Le Infezioni in Medicina. 2022; 30: 180–193.
121. Collado MC, Salminen S, Vinderola G. Postbiotics: Defining the impact of inactivated microbes and their metabolites on promotion of health. Academic Press; 2021. pp. 257–268.
122. Lahtinen SJ. Probiotic viability—Does it matter. Microbial Ecology in Health and Disease. 2012; 23: 10–14.
123. Inoue Y, Kambara T, Murata N, et al. Effects of oral administration of Lactobacillus acidophilus L-92 on the symptoms and serum cytokines of atopic dermatitis in Japanese adults: A double-blind, randomized, clinical trial. International Archives of Allergy and Immunology. 2014; 165: 247–254.
124. Bourebaba Y, Marycz K, Mularczyk M, Bourebaba L. Postbiotics as potential new therapeutic agents for metabolic disorders management. Biomedicine & Pharmacotherapy. 2022; 153: 113138.
125. Kang J, Lee JJ, Cho JH, et al. Effects of dietary inactivated probiotics on growth performance and immune responses of weaned pigs. Journal of Animal Science and Technology. 2021; 63: 520–530.
126. Jensen G, Benson K, Carter S, Endres J. GanedenBC30™ cell wall and metabolites: Anti-inflammatory and immune modulating effects in vitro. BMC Immunology. 2010; 11: 15.
127. Escamilla J, Lane MA, Maitin V. Cell-free supernatants from probiotic Lactobacillus casei and Lactobacillus rhamnosus GG decrease colon cancer cell invasion in vitro. Nutrition and Cancer. 2012; 64: 871–878.
128. Cousin FJ, Jouan-Lanhouet S, Dimanche-Boitrel MT, et al. Milk fermented by Propionibacterium freudenreichii induces apoptosis of HGT-1 human gastric cancer cells. PLoS One. 2012; 7: e31892.
129. Maghsood F, Mirshafiey A, Farahani MM, et al. Dual effects of cell free supernatants from Lactobacillus acidophilus and Lactobacillus rhamnosus GG in regulation of MMP-9 by up-regulating TIMP-1 and down-regulating CD147 in PMA-differentiated THP-1 cells. Cell. 2018; 19: 559–566.
130. Wang S, Ahmadi S, Nagpal R, et al. Lipoteichoic acid from the cell wall of a heat killed Lactobacillus paracasei D3-5 ameliorates aging-related leaky gut, inflammation and improves physical and cognitive functions: From C. elegans to mice. Geroscience. 2020; 42: 333–352.
131. Martorell P, Alvarez B, Llopis S, et al. Heat-treated Bifidobacterium longum CECT-7347: A whole-cell postbiotic with antioxidant, anti-inflammatory, and gut-barrier protection properties. Antioxidants. 2021; 10; 536.
132. Schiavi E, Gleinser M, Molloy E, et al. The surface-associated exopolysaccharide of Bifidobacterium longum 35624 plays an essential role in dampening host proinflammatory responses and repressing local TH17 responses. Applied Environmental Microbiology. 2016; 82: 7185–7196.
133. Sungur T, Aslim B, Karaaslan C, Aktas B. Impact of exopolysaccharides (EPSs) of Lactobacillus gasseri strains isolated from human vagina on cervical tumor cells (HeLa). Anaerobe. 2017; 47: 137–144.
134. Compare D, Rocco A, Coccoli P, et al. Lactobacillus casei DG and its postbiotic reduce the inflammatory mucosal response: An ex-vivo organ culture model of post-infectious irritable bowel syndrome. BMC Gastroenterology. 2017; 17: 53.
135. Hao H, Zhang X, Tong L, et al. Effect of extracellular vesicles derived from Lactobacillus plantarum Q7 on gut microbiota and ulcerative colitis in mice. Frontiers in Immunology. 2021; 12: 777147.
136. Khodaii Z, Ghaderian SMH, Natanzi MM. Probiotic bacteria and their supernatants protect enterocyte cell lines from enteroinvasive Escherichia coli (EIEC) invasion. International Journal of Molecular and Cellular Medicine. 2017; 6: 183–189.
137. Singh TP, Kaur G, Kapila S, Malik RK. Antagonistic activity of Lactobacillus reuteri strains on the adhesion characteristics of selected pathogens. Frontiers in Microbiology. 2017; 8: 486.
138. Gao J, Li Y, Wan Y, et al. Novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Frontiers in Microbiology. 2019; 10: 477.
139. Mi XJ, Tran THM, Park HR, et al. Immune-enhancing effects of postbiotic produced by Bacillus velezensis Kh2-2 isolated from Korea foods. Food Research International. 2021; 152: 110911.
140. Gurunathan S, Thangaraj P, Das J, Kim JH. Antibacterial and antibiofilm effects of Pseudomonas aeruginosa-derived outer membrane vesicles against Streptococcus mutans. Heliyon. 2023b; 9: e22606.
141. Inturri R, Molinaro A, Di Lorenzo F, et al. Chemical and biological properties of the novel exopolysaccharide produced by a probiotic strain of Bifidobacterium longum. Carbohydrate Polymers. 2017b; 174: 1172–1180.
142. Jensen GS, Cash HA, Farmer S, Keller D. Inactivated probiotic Bacillus coagulans GBI-30 induces complex immune activating, anti-inflammatory, and regenerative markers in vitro. Journal of Inflammation Research. 2017; 10: 107–117.
143. Liang TW, Tseng SC, Wang SL. Production and characterization of antioxidant properties of exopolysaccharide(s) from Peanibacillus mucilaginosus TKU032. Drugs. 2016; 14: 40.
144. Hoarau C, Martin L, Faugaret D, et al. Supernatant from Bifidobacterium differentially modulates transduction signaling pathways for biological functions of human dendritic cells. PLoS One. 2008; 3: e2753.
145. Wang J, Wu T, Fang X, et al. Characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus plantarum JLK0142 isolated from fermented dairy tofu. International Journal of Biological Macromolecules. 2018; 115: 985–993.
146. Jeong M, Kim JH, Lee JS, et al. Heat-Killed Lactobacillus brevis enhances phagocytic activity and generates immune-stimulatory effects through activating the TAK1 pathway. Journal of Microbiology and Biotechnology. 2020; 30: 1395–1403.
147. Maehata H, Arai S, Iwabuchi N, Abe F. Immuno-modulation by heat-killed Lacticaseibacillus paracasei MCC1849 and its application to food products. International Journal of Immunopathology & Pharmacology. 2021; 35: 1–9.
148. Nakai H, Murosaki S, Yamamoto Y, et al. Safety and efficacy of using heat-killed Lactobacillus plantarum L-137: High-dose and long-term use effects on immune-related safety and intestinal bacterial flora. Journal of Immunotoxicology. 2021; 18: 127–135.
149. Inturri R, Mangano K, Santagati M, et al. Immunomodulatory effects of Bifidobacterium longum W11 produced exopolysaccharide on cytokine production. Current Pharmaceutical Biotechnology. 2017a; 18: 883–889.
150. Kolling Y, Salva S, Villena J, Alvarez S. Are the immunomodulatory properties of Lactobacillus rhamnosus CRL1505 peptidoglycan common for all Lactobacilli during respiratory infection in malnourished mice? PLoS One. 2018; 13: e0194034.
151. Nobutani K, Sawada D, Fujiwara S, et al. The effects of administration of the Lactobacillus gasseri strain CP2305 on quality of life, clinical symptoms and changes in gene expression in patients with irritable bowel syndrome. Journal of Applied Microbiology. 2017; 122: 212–224.
152. Nishida K, Sawada D, Kuwano Y, et al. Health benefits of Lactobacillus gasseri CP2305 tablets in young adults exposed to chronic stress: A randomized, double-blind, placebo-controlled study. Nutrients. 2019; 11: 1859.
153. Andresen V, Gschossmann J, Layer P. Heat-inactivated Bifidobacterium bifidum MIMBb75 (SYN-HI-001) in the treatment of irritable bowel syndrome: A multicentre, randomised, double-blind, placebo-controlled clinical trial. Lancet Gastroenterology Hepatology. 2020; 5: 658–666.
154. Othman MB, Sakamoto K. Effect of inactivated Bifidobacterium longum intake on obese diabetes model mice (TSOD). Food Research Intnational. 2020; 129: 108792.
155. Ghoneim MAM, Hassan AI, Mahmoud MG, Asker MS. Effect of polysaccharide from Bacillus subtilis sp. on cardiovascular diseases and atherogenic indices in diabetic rats. BMC Complementary and Alternative Medicine. 2016; 16: 112.
156. Dahech I, Belghith KS, Hamden K, et al. Antidiabetic activity of levan polysaccharide in alloxan-induced diabetic rats. International Journal of Biological Macromolecules. 2011; 49: 742–746.
157. Jensen BAH, Holm JB, Larsen IS, et al. Lysates of methylococcuscapsulatus bath induce a lean-like microbiota, intestinal FoxP3 + RORγt + IL-17 + Tregs and improve metabolism. Nature Communications. 2021; 12: 1093.
158. Bhat B, Bajaj BK. Hypocholesterolemic potential and bioactivity spectrum of an exopolysaccharide from a probiotic isolate Lactobacillus paracasei M7. Bioactive Carbohydrates and Dietary Fibre. 2019; 19: 100191.
159. Osman A, El-Gazzar N, Almanaa TN, et al. Lipolytic postbiotic from Lactobacillus paracasei manages metabolic syndrome in albino Wistar rats. Molecules. 2021; 26: 472.
160. Balaguer F, Enrique M, Llopis S, et al. Lipoteichoic acid from Bifidobacterium animalis subsp. lactis BPL1: A novel postbiotic that reduces fat deposition via IGF-1 pathway. Microbial Biotechnology. 2021; 15: 805–816.
161. Depommier C, Van Hul M, Everard A, et al. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes. 2020; 11: 1231–1245.
162. Chen D, Jin D, Huang S, et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Letters. 2020; 469: 456–467.
163. Lee JE, Lee J, Kim JH, et al. Characterization of the anti-cancer activity of the probiotic bacterium Lactobacillus fermentum using 2D vs. 3D Culture in colorectal cancer cells. Biomolecules. 2019; 9: 557.
164. Gurunathan S, Ajmani A, Kim JH. Extracellular nanovesicles produced by Bacillus licheniformis: A potential anticancer agent for breast and lung cancer. Microbial Pathogenesis. 2023a; 185: 106396.
165. Rafique N, Jan SY, Dar AH, et al. Promising bioactivities of postbiotics: A comprehensive review. Journal of Agricultural and Food Research. 2023; 14: 100708.
166. Sarkar S. Whether viable and dead probiotic are equally efficacious? Nutrition & Food Science. 2018; 48: 285–300.
167. Vinderola G, Sanders ME, Salminen S, Szajewska H. Postbiotics: The concept and their use in healthy populations. Frontiers in Nutrition. 2022b; 9: 1002213.
168. Sharieff W., Bhutta Z., Schauer C., et al. Micronutrients (including zinc) reduce diarrhoea in children: The Pakistan Sprinkles Diarrhoea Study. Archives in Disease in Childhood 2006;91:573–579.
169. Kaila M, Isolauri E, Saxelin M, et al. Viable versus inactivated Lactobacillus strain GG in acute rotavirus diarrhoea. Archives of Disease in Childhood. 1995; 72: 51–53.
170. Sugahara H, Yao R, Odamaki T, Xiao JZ. Differences between live and heat-killed bifidobacteria in the regulation of immune function and the intestinal environment. Beneficial Microbes. 2017; 8: 463–472.
171. Raghavan CM, Nanda A, Yuvaraj R, et al. Assimilation of cholesterol by Lactobacillus species as probiotic. World Applied Science Journal. 2011; 14: 552–560.
172. Miremadi F, Ayyash M, Sherkat F, Stojanovska L. Cholesterol reduction mechanisms and fatty acid composition of cellular membranes of probiotic lactobacilli and bifidobacteria. Journal of Functional Foods. 2014; 9: 295–305.
173. Anila K, Kunzes A, Bhalla TC. In vitro cholesterol assimilation and functional enzymatic activities of putative probiotic Lactobacillus sp. isolated from fermented foods/beverages of North West India. Journal of Nutrition and Food Science. 2016; 6: 467.
174. Indriyani A, Juffrie M, Setyati A. Effects of live versus heat-killed probiotics on acute diarrhea in young children. Paediatrica Indonesiana. 2012; 52: 249–254.
175. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—A double-blind, placebo-controlled study. Alimentary Pharmacology and Therapeutics. 2011; 33:1123-1132
176. Sugawara T, Sawada D, Ishida Y, et al. Regulatory effect of paraprobiotic Lactobacillus gasseri CP2305 on gut environment and function. Microbial Ecology in Health and Disease. 2016; 27: 30259.
177. Awad H, Mokhtar H, Imam, SS, et al. Comparison between killed and living probiotic usage versus placebo for the prevention of necrotizing enterocolitis and sepsis in neonates. Pakistan Journal of Biological Sciences. 2020; 15: 253–262.
178. Ashrafian F, Raftar SKA, Shahryari A, et al. Comparative effects of alive and pasteurized Akkermansia muciniphila on normal diet-fed mice. Scientific Reports. 2021; 1: 17898.
179. Geraldo BMC, Batalha MN, Milhan NVM, et al. Heat‐killed Lactobacillus reuteri and cell‐free culture supernatant have similar effects to viable probiotics during interaction with Porphyromonas gingivalis. Journal of Periodontal Research. 2020; 55: 215–220.
180. Xiao SD, Zhang de Z, Lu H, et al. Multicenter, randomized, controlled trial of heat-killed Lactobacillus acidophilus LB in patients with chronic diarrhea. Advances in Therapy. 2003; 20: 253–260.
181. Jang HJ, Song MW, Lee NK, Paik HD. Antioxidant effects of live and heat-killed probiotic Lactobacillus plantarum Ln1 isolated from kimchi. Journal Food Science and Technology. 2018; 55: 3174–3180.
182. Zolkiewicz J, Marzec A, Ruszczynski M, Feleszko W. Postbiotics—A step beyond pre- and probiotics. Nutrients. 2020; 12: 2189.
183. Gonzalez A, Galvez N, Martín J, et al. Identification of the key excreted molecule by Lactobacillus fermentum related to host iron absorption. Food Chemistry. 2017; 228: 374–380.
184. Korcok DJ, Trsic-Milanovic NA, Ivanovic ND, Dordevic BI. Development of probiotic formulation for the treatment of iron deficiency anemia. Chemical and Pharmaceutical Bulletin. 2018; 66: 347–352.
185. Konstantinov S.R., Kuipers E.J., Peppelenbosch M.P. Functional genomic analyses of the gut microbiota for CRC screening. Nature Reviews: Gastroenterology & Hepatology. 2013;10:741.
186. Liu Y, Gibson GR, Walton GE. An In vitro approach to study effects of prebiotics and probiotics on the faecal microbiota and selected immune parameters relevant to the elderly. PLoS One. 2016; 11: e0162604.
187. Piqué N, Miñana-Galbis D, Merino S, Tomás JM. The lipopolysaccharide of Aeromonas spp: Structure-activity relationships. Current Topic in Biochemical Research. 2013; 15: 41–56.
188. Bomko T, Martynov A, Nosalskaya T. Perspective of tinalized microorganisms in the development of safe probiotics. Annals of Mechnikov’s Institute. 2021; [1]: 6–14.
189. Shigwedha N, Zhang L, Sichel L, et al. More than a few lab alleviate common allergies: Impact of paraprobiotics in comparison to probiotical live cells. Journal of Biosciences and Medicines. 2014; 2: 56–64.
190. Ma L, Tu H, Chen T. Postbiotics in human health: A narrative review. Nutrients. 2023; 15: 291.
191. Iaconelli C, Lemetais G, Kechaou N, et al. Drying process strongly affects probiotics viability and functionalities. Journal of Biotechnology. 2015; 214: 17–26.
192. Charoux CMG, Free L, Hinds LM, et al. Effect of non-thermal plasma technology on microbial inactivation and total phenolic content of a model liquid food system and black pepper grains. LWT. 2020; 118: 108716.
193. Thibault H, Aubert-Jacquin C, Goulet O. Effects of long-term consumption of a fermented infant formula (with Bifidobacterium breve c50 and Streptococcus thermophilus 065) on acute diarrhea in healthy infants. Journal of Pediatric Gastroenterology and Nutrition. 2004; 39: 147–152.
194. Malagon-Rojas JN, Mantziari A, Salminen S, Szajewska H. Postbiotics for preventing and treating common infectious diseases in children: A systematic review. Nutrients. 2020; 12: 389.
195. Assmann G, Buono P, Daniele A, et al. Functional foods and cardiometabolic diseases. Nutrition, Metabolism and Cardiovascular Diseases. 2014; 24: 1272–1300.
196. Sarkar S. Efficacy of dead probiotic cells. International Journal of Food Science Nutrition & Dietetics. 2016; 5: 1.
197. Ouwehand AC, Kirjavainen PV, Shortt C, Salminen S. Probiotics: Mechanisms and established effects. International Dairy Journal. 1999; 9: 43–52.
198. O’Brien J, Crittenden R, Ouwehand AC, Salminen S. Safety evaluation of probiotics. Trends in Food Science & Technology. 1999; 10: 418–424.
199. Sanders ME, Klaenhammer TR, Ouwehand AC, et al. Effects of genetic, processing, or product formulation changes on efficacy and safety of probiotics. Annals of the New York Academy of Sciences. 2014; 1309: 1–18.
200. Tripathi MK, Giri SK. Probiotic functional foods: Survival of probiotics during processing and storage. Journal of Functional Food. 2014; 9: 225–241.
201. Mcfarland LV, Evans CT, Goldstein EJ. Strain-specificity and disease-specificity of probiotic efficacy: A systematic review and meta-analysis. Frontiers in Medicine. 2018; 5: 124.
202. Azais-Braesco V, Bresson JL, Guarner F, Corthier G. Not all lactic acid bacteria are probiotics, ...but some are. British Journal of Nutrition. 2010; 103: 1079–1081.
203. Ansari JM, Colasacco C, Emmanouil E, et al. Strain-level diversity of commercial probiotic isolates of Bacillus, Lactobacillus, and Saccharomyces species illustrated by molecular identification and phenotypic profiling. PLoS One. 2019; 14: e021384.
204. Lenoir-Wijnkoop I, Gerlier L, Bresson JL, et al. Public health and budget impact of probiotics on common respiratory tract infections: A modelling study. PLoS One. 2015; 10: e0122765.
205. Gueimonde M, Sanchez B, Margolleas A. Antibiotic resistance in probiotic bacteria. Frontiers in Microbiology. 2013; 4: 202.
206. Sanders ME, Akkermans LM, Haller D, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010; 1: 164–185.
207. Patel AK, Singhania RP, Pandey A, Chincholkar SB. Probiotic bile salt hydrolase: Current development and perspectives. Applied Biochemistry and Biotechnology. 2010; 162: 66–88.
208. Woo J, Ahn J. Probiotic-mediated competition, exclusion and displacement in biofilm formation by food-borne pathogens. Letters in Applied Microbiology. 2013; 56: 307–313.
209. Monteagudo-Mera A, Rastall RA, Gibson GR, et al. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Applied Microbiology & Biotechnology. 2019; 103: 6463–6472.
210. Reid G, Gadir AA, Dhir R. Probiotics: Reiterating what they are and what they are not. Frontiers in Microbiology. 2019; 10: 424.




.jpg)
.jpg)

