Oxidative and hydrolytic deteriorations of lipids and several alternative pathways for their protections: An overview
DOI:
https://doi.org/10.18686/fnc238Keywords:
food; stability; antioxidants; bioactive packaging; shelf-lifeAbstract
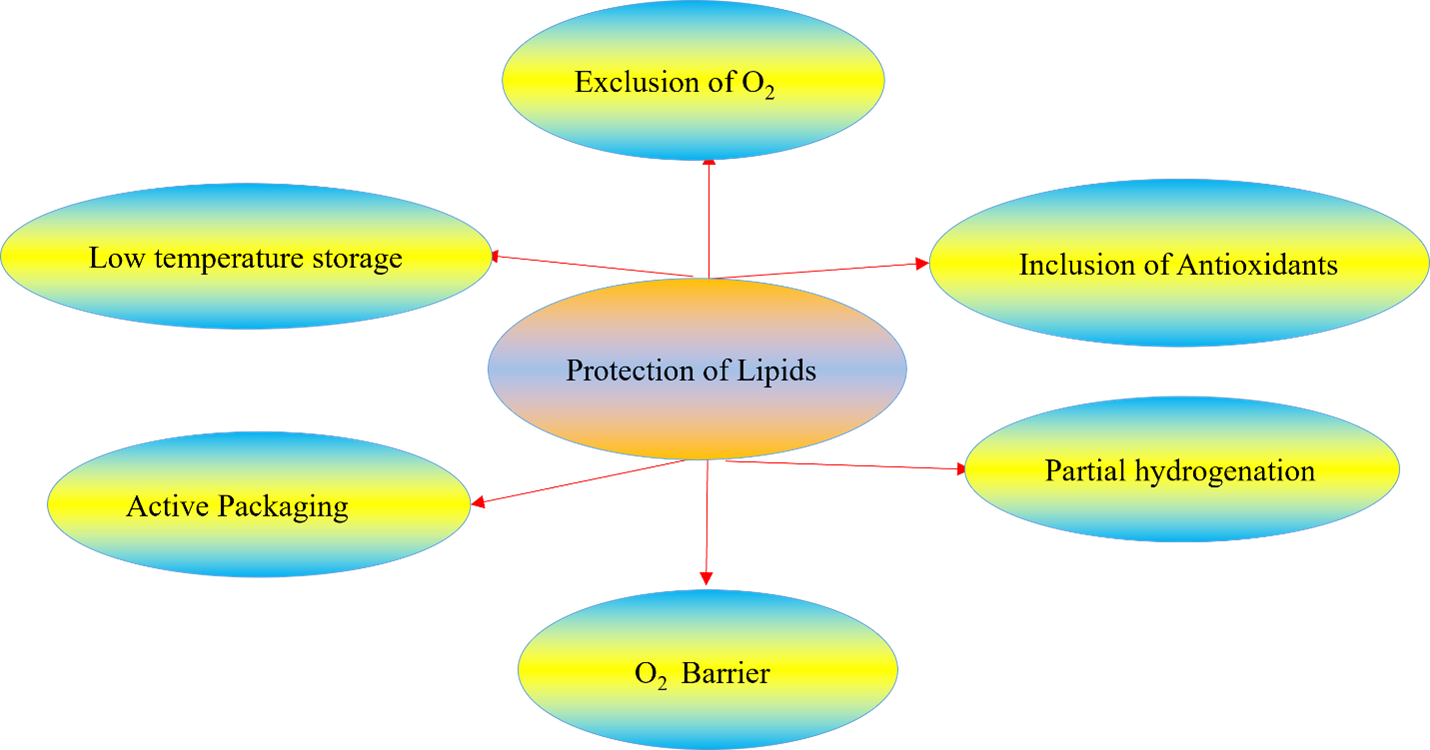
Human beings need macronutrients (lipids, carbohydrates, and proteins) in their diets. Among them, lipids are more susceptible to oxidative deteriorations. Oxidation and hydrolysis are two major lipid deterioration reactions that occurred during their processing and storage. This article provided an overview of major deteriorations of lipids and several pathways for their protection. The following conclusions were made: (i) oxidation and hydrolysis of lipids result in chemical, physical, nutritional and quality changes; (ⅱ) the oxidation rate varied by level of oxygen, composition of fatty acids, the number of double bonds, the locations of double bonds in the fatty acid chains of triacylglycerides, the nature of the molecular surface exposed to O2, the conditions for processing or storage, and the activity of pro- and antioxidants; (ⅲ) study on the kinetics of reactions helps in the understanding of the deteriorations; (ⅳ) several pathways were used to improve the stability or suppress/reduce lipid deterioration; (v) the deterioration can be reduced by exclusion of oxygen, incorporation of antioxidants, storage at low temperature, partial hydrogenation of unsaturated lipids, incorporation of bioactive or oxygen barrier compounds in food packaging systems; and (ⅵ) natural antioxidants are safe and unique alternatives to synthetic ones. They have the potential to protect both foodstuffs and human beings from several diseases arising from oxidative processes.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Author(s)

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
1. Belitz HD, Grosch W, Schieberle P. Food Chemistry. Berlin: Springer-Verlag; 2009.
2. Kim HJ, Min DB. Chemistry of lipid oxidation. In: Akoh CC, Min DB (editors). Food Lipids: Chemistry, Nutrition, and Biotechnology. CRC Press, Taylor & Francis Group, New York; 2008. pp. 299-320.
3. Logan A, Nienabar U, Pan X. Lipid Oxidation: Challenges in Food Systems. Elsevier: Amsterdam; 2013.
4. Waraho T, McClements DJ, Decker EA. Mechanisms of lipid oxidation in food dispersions. Trends in Food Science & Technology. 2011; 22(1): 3-13. doi: 10.1016/j.tifs.2010.11.003 DOI: https://doi.org/10.1016/j.tifs.2010.11.003
5. Mozuraityte R, Kristinova V, Rustad T. Oxidation of Food Components. In: Encyclopedia of Food and Health. Elsevier; 2016. pp. 186-190. DOI: https://doi.org/10.1016/B978-0-12-384947-2.00508-0
6. Vieira SA, Zhang G, Decker EA. Biological Implications of Lipid Oxidation Products. Journal of the American Oil Chemists’ Society. 2017; 94(3): 339-351. doi: 10.1007/s11746-017-2958-2 DOI: https://doi.org/10.1007/s11746-017-2958-2
7. Bhat ZF, Bhat HF, Manzoor M, et al. Enhancing the lipid stability of foods of animal origin using edible packaging systems. Food Chemistry: X. 2024; 21: 101185. doi: 10.1016/j.fochx.2024.101185 DOI: https://doi.org/10.1016/j.fochx.2024.101185
8. Tatiyaborworntham N, Yin J, Richards MP. Factors influencing the antioxidant effect of phospholipase A2 against lipid oxidation promoted by trout hemoglobin and hemin in washed muscle. Food Chemistry. 2021; 343: 128428. doi: 10.1016/j.foodchem.2020.128428 DOI: https://doi.org/10.1016/j.foodchem.2020.128428
9. Amaral AB, Silva MV da, Lannes SC da S. Lipid oxidation in meat: mechanisms and protective factors – a review. Food Science and Technology. 2018; 38(suppl 1): 1-15. doi: 10.1590/fst.32518 DOI: https://doi.org/10.1590/fst.32518
10. Barden L, Decker EA. Lipid Oxidation in Low-moisture Food: A Review. Critical Reviews in Food Science and Nutrition. 2013; 56(15): 2467-2482. doi: 10.1080/10408398.2013.848833 DOI: https://doi.org/10.1080/10408398.2013.848833
11. Wang D, Xiao H, Lyu X, et al. Lipid oxidation in food science and nutritional health: A comprehensive review. Oil Crop Science. 2023; 8(1): 35-44. doi: 10.1016/j.ocsci.2023.02.002 DOI: https://doi.org/10.1016/j.ocsci.2023.02.002
12. Bonilla J, Sobral PJA. Investigation of the physicochemical, antimicrobial and antioxidant properties of gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Bioscience. 2016; 16: 17-25. doi: 10.1016/j.fbio.2016.07.003 DOI: https://doi.org/10.1016/j.fbio.2016.07.003
13. Jridi M, Hajji S, Ayed HB, et al. Physical, structural, antioxidant and antimicrobial properties of gelatin–chitosan composite edible films. International Journal of Biological Macromolecules. 2014; 67: 373-379. doi: 10.1016/j.ijbiomac.2014.03.054 DOI: https://doi.org/10.1016/j.ijbiomac.2014.03.054
14. Flores-López ML, Cerqueira MA, de Rodríguez DJ, et al. Perspectives on Utilization of Edible Coatings and Nano-laminate Coatings for Extension of Postharvest Storage of Fruits and Vegetables. Food Engineering Reviews. 2015; 8(3): 292-305. doi: 10.1007/s12393-015-9135-x DOI: https://doi.org/10.1007/s12393-015-9135-x
15. Bear MF, Connors BW, Paradiso MA. Neuroscience, Exploring the brain, 2nd ed. Lippincott Williams & Wilkins: Baltimore; 2001.
16. Raven PH, Johnson GB. Biology, 6th ed. McGraw Hill, Boston; 2002.
17. Damerau A, Ahonen E, Kortesniemi M, et al. Docosahexaenoic acid in regio- and enantiopure triacylglycerols: Oxidative stability and influence of chiral antioxidant. Food Chemistry. 2023; 402: 134271. doi: 10.1016/j.foodchem.2022.134271 DOI: https://doi.org/10.1016/j.foodchem.2022.134271
18. Toorani MR, Golmakani MT. Effect of triacylglycerol structure on the antioxidant activity of γ-oryzanol. Food Chemistry. 2022; 370: 130974. doi: 10.1016/j.foodchem.2021.130974 DOI: https://doi.org/10.1016/j.foodchem.2021.130974
19. Zhu W, Han M, Bu Y, et al. Plant polyphenols regulating myoglobin oxidation and color stability in red meat and certain fish: A review. Critical Reviews in Food Science and Nutrition. 2022; 64(8): 2276-2288. doi: 10.1080/10408398.2022.2122922 DOI: https://doi.org/10.1080/10408398.2022.2122922
20. Chen J, Rosenthal A. Modifying Food Texture, Novel Ingredients and Processing Techniques. Woodhead Publishing, Elsevier: Amsterdam; 2015.
21. Lawless HT, Heymann H. Sensory Evaluation of Food. Springer New York; 2010. DOI: https://doi.org/10.1007/978-1-4419-6488-5
22. Wang W, Li Y, Cai L, et al. Characteristics on the oxidation stability of infant formula powder with different ingredients during storage. Food Science & Nutrition. 2020; 8(12): 6392-6400. doi: 10.1002/fsn3.1928 DOI: https://doi.org/10.1002/fsn3.1928
23. Wang X, Zeng Q, del Mar Contreras M, et al. Profiling and quantification of phenolic compounds in Camellia seed oils: Natural tea polyphenols in vegetable oil. Food Research International. 2017; 102: 184-194. doi: 10.1016/j.foodres.2017.09.089 DOI: https://doi.org/10.1016/j.foodres.2017.09.089
24. Shahidi F, Oh WY. Lipid-derived flavor and off-flavor of traditional and functional foods: an overview. Journal of Food Bioactives. 2020; 10: 20-31. doi: 10.31665/jfb.2020.10224 DOI: https://doi.org/10.31665/JFB.2020.10224
25. Sunarharum WB, Williams DJ, Smyth HE. Complexity of coffee flavor: A compositional and sensory perspective. Food Research International. 2014; 62: 315-325. doi: 10.1016/j.foodres.2014.02.030 DOI: https://doi.org/10.1016/j.foodres.2014.02.030
26. Farbiszewski R, Kranc R. Olfactory receptors and the mechanism of odor perception. Polish Annals of Medicine. 2013; 20(1): 51-55. doi: 10.1016/j.poamed.2013.02.002 DOI: https://doi.org/10.1016/j.poamed.2013.02.002
27. Marcus JB. Aging, Nutrition and Taste: Nutrition, Food Science and Culinary Perspectives for Aging Tastefully. Academic Press: London; 2019.
28. Porcherot C, Delplanque S, Gaudreau N, et al. Seeing, smelling, feeling! Is there an influence of color on subjective affective responses to perfumed fabric softeners? Food Quality and Preference. 2013; 27(2): 161-169. doi: 10.1016/j.foodqual.2012.06.011 DOI: https://doi.org/10.1016/j.foodqual.2012.06.011
29. Pegg RB, Shahidi F. Nitrite curing of meat; The N-Nitroso-amine Problem and Nitrite Alternatives. Food & Nutrition Press, Trumball, CT; 2000. pp. 105-132.
30. FAO and WHO. Food Safety Risk Analysis: A guide for national food safety Authorities. World Health Organization, Food and Agriculture Organization of the United Nations, Rome; 2006.
31. Andersen V, Motarjemi Y. Food Safety Management: A Practical Guide for the Food Industry. Academic Press, London; 2023. DOI: https://doi.org/10.1016/B978-0-12-820013-1.00046-2
32. Nordhagen S, Lambertini E, DeWaal CS, et al. Integrating nutrition and food safety in food systems policy and programming. Global Food Security. 2022; 32: 100593. doi: 10.1016/j.gfs.2021.100593 DOI: https://doi.org/10.1016/j.gfs.2021.100593
33. Lawrence AJ. Recovery, refining, converting, and stabilizing edible fats and oils. In: Akoh CC, Min DB (editors). Food Lipids: Chemistry, Nutrition, and Biotechnology. CRC Press, Taylor & Francis Group, New York; 2008. pp. 205-247.
34. Domínguez R, Pateiro M, Gagaoua M, et al. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants. 2019; 8(10): 429. doi: 10.3390/antiox8100429 DOI: https://doi.org/10.3390/antiox8100429
35. Ghnimi S, Budilarto E, Kamal‐Eldin A. The New Paradigm for Lipid Oxidation and Insights to Microencapsulation of Omega‐3 Fatty Acids. Comprehensive Reviews in Food Science and Food Safety. 2017; 16(6): 1206-1218. doi: 10.1111/1541-4337.12300 DOI: https://doi.org/10.1111/1541-4337.12300
36. Marković ZS, Milenković DA. Different theoretical approaches in the study of antioxidative mechanisms. Computational Modeling in Bioengineering and Bioinformatics. Published online 2020: 211-256. doi: 10.1016/b978-0-12-819583-3.00007-2 DOI: https://doi.org/10.1016/B978-0-12-819583-3.00007-2
37. Kuhn H, Banthiya S, van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2015; 1851(4): 308-330. doi: 10.1016/j.bbalip.2014.10.002 DOI: https://doi.org/10.1016/j.bbalip.2014.10.002
38. Valgimigli L. Lipid Peroxidation and Antioxidant Protection. Biomolecules. 2023; 13(9): 1291. doi: 10.3390/biom13091291 DOI: https://doi.org/10.3390/biom13091291
39. Diaz-Uribe C, Vallejo W, De la Hoz T, et al. Theoretical and kinetic study of the singlet oxygen quenching reaction by hesperidin isolated from mandarin (Citrus reticulata) fruit peels. Chemical Papers. 2021; 76(1): 169-178. doi: 10.1007/s11696-021-01825-2 DOI: https://doi.org/10.1007/s11696-021-01825-2
40. Van Dyck S. The impact of singlet oxygen on lipid oxidation in foods. Oxidation in Foods and Beverages and Antioxidant Applications; 2010. DOI: https://doi.org/10.1533/9780857090447.1.57
41. Foret MK, Lincoln R, Do Carmo S, et al. Connecting the “Dots”: From Free Radical Lipid Autoxidation to Cell Pathology and Disease. Chemical Reviews. 2020; 120(23): 12757-12787. doi: 10.1021/acs.chemrev.0c00761 DOI: https://doi.org/10.1021/acs.chemrev.0c00761
42. Ito J, Shimizu N, Kobayashi E, et al. A novel chiral stationary phase LC-MS/MS method to evaluate oxidation mechanisms of edible oils. Scientific Reports. 2017; 7(1). doi: 10.1038/s41598-017-10536-2 DOI: https://doi.org/10.1038/s41598-017-10536-2
43. Kasaai MR. Milk fat: A review of chemical, physical, and nutritional aspects. In: Recent Research and Development of Agricultural and Food Chemistry. Research Signpost; 2005.
44. Skibsted LH. Light induced changes in dairy products. In: Packaging of milk products: Bulletin of the International Dairy Federation. 2000; 346: 3-9.
45. Feussner I, Wasternack C. The lipoxygenase pathway. Annual Review of Plant Biology. 2002; 53(1): 275-297. doi: 10.1146/annurev.arplant.53.100301.135248 DOI: https://doi.org/10.1146/annurev.arplant.53.100301.135248
46. Lampi AM, Yang Z, Mustonen O, et al. Potential of faba bean lipase and lipoxygenase to promote formation of volatile lipid oxidation products in food models. Food Chemistry. 2020; 311: 125982. doi: 10.1016/j.foodchem.2019.125982 DOI: https://doi.org/10.1016/j.foodchem.2019.125982
47. Hatcher E, Soudackov AV, Hammes-Schiffer S. Proton-Coupled Electron Transfer in Soybean Lipoxygenase: Dynamical Behavior and Temperature Dependence of Kinetic Isotope Effects. Journal of the American Chemical Society. 2006; 129(1): 187-196. doi: 10.1021/ja0667211 DOI: https://doi.org/10.1021/ja0667211
48. Cofrades S, López-Lopez I, Bravo L, et al. Nutritional and Antioxidant Properties of Different Brown and Red Spanish Edible Seaweeds. Food Science and Technology International. 2010; 16(5): 361-370. doi: 10.1177/1082013210367049 DOI: https://doi.org/10.1177/1082013210367049
49. Huang X, Ahn DU. Lipid oxidation and its implications to meat quality and human health. Food Science and Biotechnology. 2019; 28(5): 1275-1285. doi: 10.1007/s10068-019-00631-7 DOI: https://doi.org/10.1007/s10068-019-00631-7
50. Gumus CE, Decker EA. Oxidation in Low Moisture Foods as a Function of Surface Lipids and Fat Content. Foods. 2021; 10(4): 860. doi: 10.3390/foods10040860 DOI: https://doi.org/10.3390/foods10040860
51. Mariutti LRB, Bragagnolo N. Influence of salt on lipid oxidation in meat and seafood products: a review. Food Research International. 2017; 94: 90-100. doi: 10.1016/j.foodres.2017.02.003 DOI: https://doi.org/10.1016/j.foodres.2017.02.003
52. Ahmed M, Pickova J, Ahmad T, et al. Oxidation of Lipids in Foods. Sarhad Journal of Agriculture. 2016; 32(3): 230-238. doi: 10.17582/journal.sja/2016.32.3.230.238 DOI: https://doi.org/10.17582/journal.sja/2016.32.3.230.238
53. Yamuangmorn S, Sreethong T, Saenchai C, et al. Effects of roasting conditions on anthocyanin, total phenolic content, and antioxidant capacity in pigmented and non-pigmented rice varieties. International Food Research Journal. 2021; 28(1): 73-82. doi: 10.47836/ifrj.28.1.07 DOI: https://doi.org/10.47836/ifrj.28.1.07
54. Kocadağlı T, Göncüoğlu N, Hamzalıoğlu A, et al. In depth study of acrylamide formation in coffee during roasting: role of sucrose decomposition and lipid oxidation. Food & Function. 2012; 3(9): 970. doi: 10.1039/c2fo30038a DOI: https://doi.org/10.1039/c2fo30038a
55. Oestreich-Janzen S. Chemistry of Coffee. Comprehensive Natural Products II. 2010; 3: 1085-1117. doi: 10.1016/b978-008045382-8.00708-5 DOI: https://doi.org/10.1016/B978-008045382-8.00708-5
56. Buffo RA, Cardelli‐Freire C. Coffee flavour: an overview. Flavour and Fragrance Journal. 2004; 19(2): 99-104. doi: 10.1002/ffj.1325 DOI: https://doi.org/10.1002/ffj.1325
57. Yang N, Liu C, Liu X, et al. Determination of volatile marker compounds of common coffee roast defects. Food Chemistry. 2016; 211: 206-214. doi: 10.1016/j.foodchem.2016.04.124 DOI: https://doi.org/10.1016/j.foodchem.2016.04.124
58. Choe E, Min DB. Mechanisms and Factors for Edible Oil Oxidation. Comprehensive Reviews in Food Science and Food Safety. 2006; 5(4): 169-186. doi: 10.1111/j.1541-4337.2006.00009.x DOI: https://doi.org/10.1111/j.1541-4337.2006.00009.x
59. McClements DJ, Decker EA. Lipids. In: Damodaran S, Parkin KL, Fennema OR (editors). Food Chemistry. CRC Press, Boca Raton, FL, USA; 2007. pp. 155-216.
60. Shahidi F. Lipid-derived flavors in meat products. Meat Processing; 2002. DOI: https://doi.org/10.1201/9781439823163.ch5
61. Shahidi F, Zhong Y. Measurement of antioxidant activity. Journal of Functional Foods. 2015; 18: 757-781. doi: 10.1016/j.jff.2015.01.047 DOI: https://doi.org/10.1016/j.jff.2015.01.047
62. Berg JM, Tymoczko JL, Stryer L. Biochemistry, 5th ed. W.H. Freeman and Company: New York; 2002.
63. Jerónimo E, Soldado D, Sengo S, et al. Increasing the α-tocopherol content and lipid oxidative stability of meat through dietary Cistus ladanifer L. in lamb fed increasing levels of polyunsaturated fatty acid rich vegetable oils. Meat Science. 2020; 164: 108092. doi: 10.1016/j.meatsci.2020.108092 DOI: https://doi.org/10.1016/j.meatsci.2020.108092
64. Canistro D, Boccia C, Falconi R, et al. Redox-Based Flagging of the Global Network of Oxidative Stress Greatly Promotes Longevity. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2014; 70(8): 936-943. doi: 10.1093/gerona/glu160 DOI: https://doi.org/10.1093/gerona/glu160
65. Miyamoto S, Martinez GR, Medeiros MHG, et al. Singlet molecular oxygen generated by biological hydroperoxides. Journal of Photochemistry and Photobiology B: Biology. 2014; 139: 24-33. doi: 10.1016/j.jphotobiol.2014.03.028 DOI: https://doi.org/10.1016/j.jphotobiol.2014.03.028
66. Pratt DA, Tallman KA, Porter NA. Free Radical Oxidation of Polyunsaturated Lipids: New Mechanistic Insights and the Development of Peroxyl Radical Clocks. Accounts of Chemical Research. 2011; 44(6): 458-467. doi: 10.1021/ar200024c DOI: https://doi.org/10.1021/ar200024c
67. Valgimigli L, Pratt DA. Antioxidants in Chemistry and Biology. WILEY; 2012. DOI: https://doi.org/10.1002/9781119953678.rad055
68. Zielinski ZAM, Pratt DA. Lipid Peroxidation: Kinetics, Mechanisms, and Products. The Journal of Organic Chemistry. 2017; 82(6): 2817-2825. doi: 10.1021/acs.joc.7b00152 DOI: https://doi.org/10.1021/acs.joc.7b00152
69. Bursal E, Köksal E, Gülçin İ, et al. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium L.) determined by LC–MS/MS. Food Research International. 2013; 51(1): 66-74. doi: 10.1016/j.foodres.2012.11.022 DOI: https://doi.org/10.1016/j.foodres.2012.11.022
70. Benhabiles MS, Salah R, Lounici H, et al. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocolloids. 2012; 29(1): 48-56. doi: 10.1016/j.foodhyd.2012.02.013 DOI: https://doi.org/10.1016/j.foodhyd.2012.02.013
71. Zhang J jing, Tu Z cai, Wang H, et al. Mechanism of the effect of 2, 2′-azobis (2-amidinopropane) dihydrochloride simulated lipid oxidation on the IgG/IgE binding ability of ovalbumin. Food Chemistry. 2020; 327: 127037. doi: 10.1016/j.foodchem.2020.127037 DOI: https://doi.org/10.1016/j.foodchem.2020.127037
72. Guéraud F, Atalay M, Bresgen N, et al. Chemistry and biochemistry of lipid peroxidation products. Free Radical Research. 2010; 44(10): 1098-1124. doi: 10.3109/10715762.2010.498477 DOI: https://doi.org/10.3109/10715762.2010.498477
73. Jackson V, Penumetcha M. Dietary oxidised lipids, health consequences and novel food technologies that thwart food lipid oxidation: an update. International Journal of Food Science & Technology. 2018; 54(6): 1981-1988. doi: 10.1111/ijfs.14058 DOI: https://doi.org/10.1111/ijfs.14058
74. Kloska A, Węsierska M, Malinowska M, et al. Lipophagy and Lipolysis Status in Lipid Storage and Lipid Metabolism Diseases. International Journal of Molecular Sciences. 2020; 21(17): 6113. doi: 10.3390/ijms21176113 DOI: https://doi.org/10.3390/ijms21176113
75. Xie M, Dong X, Yu Y, et al. A novel method for detection of lipid oxidation in edible oil. LWT. 2020; 123: 109068. doi: 10.1016/j.lwt.2020.109068 DOI: https://doi.org/10.1016/j.lwt.2020.109068
76. Warner K, Neff WE, Byrdwell WC, et al. Effect of Oleic and Linoleic Acids on the Production of Deep-Fried Odor in Heated Triolein and Trilinolein. Journal of Agricultural and Food Chemistry. 2000; 49(2): 899-905. doi: 10.1021/jf000822f DOI: https://doi.org/10.1021/jf000822f
77. Shahidi F, Zhong Y. Lipid oxidation and improving the oxidative stability. Chemical Society Reviews. 2010; 39(11): 4067. doi: 10.1039/b922183m DOI: https://doi.org/10.1039/b922183m
78. Rinaldi S, Palocci G, Contò M, et al. Chemical characteristics, oxidation and proteolysis in cheese produced from fresh or stored milk subjected to heat treatments. International Journal of Dairy Technology. 2023; 76(3): 638-649. doi: 10.1111/1471-0307.12947 DOI: https://doi.org/10.1111/1471-0307.12947
79. Thierry A, Collins YF, Abeijón Mukdsi MC, et al. Lipolysis and Metabolism of Fatty Acids in Cheese. Cheese, 4th ed. Academic Press; 2017. DOI: https://doi.org/10.1016/B978-0-12-417012-4.00017-X
80. Jamróz E, Tkaczewska J, Zając M, et al. Utilisation of soybean post-production waste in single- and double-layered films based on furcellaran to obtain packaging materials for food products prone to oxidation. Food Chemistry. 2022; 387: 132883. doi: 10.1016/j.foodchem.2022.132883 DOI: https://doi.org/10.1016/j.foodchem.2022.132883
81. Kasaai MR. Bio-nano-composites containing at least two components, chitosan and zein, for food packaging applications: A review of the nano-composites in comparison with the conventional counterparts. Carbohydrate Polymers. 2022; 280: 119027. doi: 10.1016/j.carbpol.2021.119027 DOI: https://doi.org/10.1016/j.carbpol.2021.119027
82. El-Sayed HS, El-Sayed SM, Mabrouk AMM, et al. Development of Eco-friendly Probiotic Edible Coatings Based on Chitosan, Alginate and Carboxymethyl Cellulose for Improving the Shelf Life of UF Soft Cheese. Journal of Polymers and the Environment. 2021; 29(6): 1941-1953. doi: 10.1007/s10924-020-02003-3 DOI: https://doi.org/10.1007/s10924-020-02003-3
83. Liang J, Yan H, Wang X, et al. Encapsulation of epigallocatechin gallate in zein/chitosan nanoparticles for controlled applications in food systems. Food Chemistry. 2017; 231: 19-24. doi: 10.1016/j.foodchem.2017.02.106 DOI: https://doi.org/10.1016/j.foodchem.2017.02.106
84. Schreiber SB, Bozell JJ, Hayes DG, et al. Introduction of primary antioxidant activity to chitosan for application as a multifunctional food packaging material. Food Hydrocolloids. 2013; 33(2): 207-214. doi: 10.1016/j.foodhyd.2013.03.006 DOI: https://doi.org/10.1016/j.foodhyd.2013.03.006
85. Na X, Zou B, Zheng X, et al. One-step spraying of protein-anchored chitosan oligosaccharide antimicrobial coating for food preservation. International Journal of Biological Macromolecules. 2024; 275: 133330. doi: 10.1016/j.ijbiomac.2024.133330 DOI: https://doi.org/10.1016/j.ijbiomac.2024.133330
86. Nowzari F, Shábanpour B, Ojagh SM. Comparison of chitosan–gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chemistry. 2013; 141(3): 1667-1672. doi: 10.1016/j.foodchem.2013.03.022 DOI: https://doi.org/10.1016/j.foodchem.2013.03.022
87. Sami R, Elhakem A, Alharbi M, et al. The combined effect of coating treatments to nisin, nano-silica, and chitosan on oxidation processes of stored button mushrooms at 4 °C. Scientific Reports. 2021; 11(1). doi: 10.1038/s41598-021-85610-x DOI: https://doi.org/10.1038/s41598-021-85610-x
88. Dua S, Bhat ZF, Kumar S. Effect of oleuropein on the oxidative stability and storage quality of Tabaq-Maz, fried mutton ribs. Food Bioscience. 2015; 12: 84-92. doi: 10.1016/j.fbio.2015.08.002 DOI: https://doi.org/10.1016/j.fbio.2015.08.002
89. Lim M, McFetridge J, Liesebach J. Frozen Food Components and Chemical Reactions. Handbook of Frozen Foods; 2004. DOI: https://doi.org/10.1201/9780203022009.pt2
90. Mahajan K, Kumar S, Bhat ZF, et al. Aloe vera and carrageenan based edible film improves storage stability of ice-cream. Applied. Food Research. 2022; 2(1): 100128. doi: 10.1016/j.afres.2022.100128 DOI: https://doi.org/10.1016/j.afres.2022.100128
91. Yam KL, Saba RG, Ho YC. Packaging: Part 1- General Consideration. In: Encyclopedia of Food Science and Technology: Second Edition. John Wiley & Sons, New York; 2000. pp. 1807- 1811.
92. Cunha LCM, Monteiro MLG, Lorenzo JM, et al. Natural antioxidants in processing and storage stability of sheep and goat meat products. Food Research International. 2018; 111: 379-390. doi: 10.1016/j.foodres.2018.05.041 DOI: https://doi.org/10.1016/j.foodres.2018.05.041
93. Lu T, Shen Y, Wang J, et al. Improving oxidative stability of flaxseed oil with a mixture of antioxidants. Journal of Food Processing and Preservation. 2019; 44(3). doi: 10.1111/jfpp.14355 DOI: https://doi.org/10.1111/jfpp.14355
94. Chien PJ, Sheu F, Huang WT, et al. Effect of molecular weight of chitosans on their antioxidative activities in apple juice. Food Chemistry. 2007; 102(4): 1192-1198. doi: 10.1016/j.foodchem.2006.07.007 DOI: https://doi.org/10.1016/j.foodchem.2006.07.007
95. Feng T, Du Y, Li J, et al. Antioxidant activity of half N-acetylated water-soluble chitosan in vitro. European Food Research and Technology. 2006; 225(1). doi: 10.1007/s00217-006-0391-0 DOI: https://doi.org/10.1007/s00217-006-0391-0
96. Olatunde OO, Benjakul S. Antioxidants from Crustaceans: A Panacea for Lipid Oxidation in Marine-Based Foods. Food Reviews International. 2020; 38(1): 1-31. doi: 10.1080/87559129.2020.1717522 DOI: https://doi.org/10.1080/87559129.2020.1717522
97. Xing R, Liu S, Guo Z, et al. Relevance of molecular weight of chitosan and its derivatives and their antioxidant activities in vitro. Bioorganic & Medicinal Chemistry. 2005; 13(5): 1573-1577. doi: 10.1016/j.bmc.2004.12.022 DOI: https://doi.org/10.1016/j.bmc.2004.12.022
98. Yan F, Yu X, Jing Y. Optimized preparation, characterization, and antioxidant activity of chitooligosaccharide–glycine Maillard reaction products. Journal of Food Science and Technology. 2017; 55(2): 712-720. doi: 10.1007/s13197-017-2982-0 DOI: https://doi.org/10.1007/s13197-017-2982-0
99. Fan Y, Yi J, Zhang Y, et al. Improved Chemical Stability and Antiproliferative Activities of Curcumin-Loaded Nanoparticles with a Chitosan Chlorogenic Acid Conjugate. Journal of Agricultural and Food Chemistry. 2017; 65(49): 10812-10819. doi: 10.1021/acs.jafc.7b04451 DOI: https://doi.org/10.1021/acs.jafc.7b04451
100. Luo Y, Zhang B, Whent M, et al. Preparation and characterization of zein/chitosan complex for encapsulation of α-tocopherol, and its in vitro controlled release study. Colloids and Surfaces B: Biointerfaces. 2011; 85(2): 145-152. doi: 10.1016/j.colsurfb.2011.02.020 DOI: https://doi.org/10.1016/j.colsurfb.2011.02.020
101. Hosseini H, Yaghoubi Hamgini E, Jafari SM, et al. Improving the oxidative stability of sunflower seed kernels by edible biopolymeric coatings loaded with rosemary extract. Journal of Stored Products Research. 2020; 89: 101729. doi: 10.1016/j.jspr.2020.101729 DOI: https://doi.org/10.1016/j.jspr.2020.101729
102. Xiong Q, Zhang M, Wang T, et al. Lipid oxidation induced by heating in chicken meat and the relationship with oxidants and antioxidant enzymes activities. Poultry Science. 2020; 99(3): 1761-1767. doi: 10.1016/j.psj.2019.11.013 DOI: https://doi.org/10.1016/j.psj.2019.11.013
103. Huang D, Ou B, Prior RL. The Chemistry behind Antioxidant Capacity Assays. Journal of Agricultural and Food Chemistry. 2005; 53(6): 1841-1856. doi: 10.1021/jf030723c DOI: https://doi.org/10.1021/jf030723c
104. Day BPF. Active Packaging. In: Food Packaging Technology. Food Packaging Technology, Blackwell Publishing, CRC Press; 2003. pp. 282-302.
105. Cacciotti I, Ciocci M, Di Giovanni E, et al. Hydrogen Sulfide-Releasing Fibrous Membranes: Potential Patches for Stimulating Human Stem Cells Proliferation and Viability under Oxidative Stress. International Journal of Molecular Sciences. 2018; 19(8): 2368. doi: 10.3390/ijms19082368 DOI: https://doi.org/10.3390/ijms19082368
106. Jomova K, Raptova R, Alomar SY, et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Archives of Toxicology. 2023; 97(10): 2499-2574. doi: 10.1007/s00204-023-03562-9 DOI: https://doi.org/10.1007/s00204-023-03562-9
107. Cheng H, Chen L, McClements DJ, et al. Recent advances in the application of nanotechnology to create antioxidant active food packaging materials. Critical Reviews in Food Science and Nutrition. 2022; 64(10): 2890-2905. doi: 10.1080/10408398.2022.2128035 DOI: https://doi.org/10.1080/10408398.2022.2128035
108. Youssef AM, El-Sayed SamahM. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydrate Polymers. 2018; 193: 19-27. doi: 10.1016/j.carbpol.2018.03.088 DOI: https://doi.org/10.1016/j.carbpol.2018.03.088
109. Dijkstra AJ. Selectivities in Partial Hydrogenation. Journal of the American Oil Chemists’ Society. 2009; 87(1): 115-117. doi: 10.1007/s11746-009-1507-z DOI: https://doi.org/10.1007/s11746-009-1507-z
110. Zhang Z, Wang Y, Ma X, et al. Characterisation and oxidation stability of monoacylglycerols from partially hydrogenated corn oil. Food Chemistry. 2015; 173: 70-79. doi: 10.1016/j.foodchem.2014.09.155 DOI: https://doi.org/10.1016/j.foodchem.2014.09.155
111. Troncoso FD, Tonetto GM. Highly stable platinum monolith catalyst for the hydrogenation of vegetable oil. Chemical Engineering and Processing - Process Intensification. 2022; 170: 108669. doi: 10.1016/j.cep.2021.108669 DOI: https://doi.org/10.1016/j.cep.2021.108669




.jpg)
.jpg)

