等离子体技术在氢能源研究中的应用进展
DOI:
https://doi.org/10.18686/cncest312关键词:
等离子体;氢能源;电解水催化剂;甲烷裂解;氨气裂解;合成氨;机器学习摘要
氢能源是实现碳中和目标的可选方案之一。在氢能源生产、运输、使用环节,等离子体技术都起到了一定的辅助作用。尤其是等离子体由可再生的电能激发产生,是产生氢能源的一种绿色可替代技术手段。本综述总结了近年来等离子体技术在氢能源领域的作用,重点介绍了等离子体在电解水制氢、裂解甲烷、裂解氨气和合成氨的作用。等离子体在辅助合成电解水催化剂的作用主要体现在为催化剂基底材料刻蚀微沟槽,制造催化剂空穴,增加原子修饰三个方面。等离子体在裂解甲烷和氨气得到氢气,合成氨三个方面的作用主要是联合催化剂提高工艺指标。鉴于等离子体制备催化剂的重要性,我们建议采用机器学习辅助高通量筛选获得最佳理论性能的催化剂结构之后,再使用等离子体针对性合成催化剂。与此同时,使用第一性原理计算阐释催化剂催化机理。
##submission.downloads##
已出版
文章引用
期
栏目
执照
版权声明
##submission.license.cc.by4.footer##参考
1. Jackson RB, Friedlingstein P, Le Quéré C, et al. Global fossil carbon emissions rebound near pre-COVID-19 levels. Environmental Research Letters. 2022; 17(3): 031001. doi: 10.1088/1748-9326/ac55b6
2. Lee H, Calvin K, Dasgupta D, et al. IPCC, 2023: Climate Change 2023: Synthesis Report. Contribution of Working Groups Ⅰ, Ⅱ and Ⅲ to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC. 2023.
3. Fulcheri L, Dames E, Rohani V. Plasma-based conversion of methane into hydrogen and carbon black. Current Opinion in Green and Sustainable Chemistry. 2024; 50: 100973. doi: 10.1016/j.cogsc.2024.100973
4. Zhang D, Chen Y, Dai H, et al. Constructing crystalline/amorphous interfaces in crystalline vanadium disulfide/amorphous molybdenum sulfide/reduced graphene oxide nanocomposites via a hydrothermal-plasma method toward efficient Hydrogen Evolution Reaction. Catalysis Today. 2023; 422: 114234. doi: 10.1016/j.cattod.2023.114234
5. Zhou M, Zou T, Wang Z, et al. Generation of dual anion vacancies on CeO2/Co4N interfaces to facilitate Hydrogen Evolution Reaction in alkaline solution. New Journal of Chemistry. 2023; 47(31): 14792–14800. doi: 10.1039/d3nj02218h
6. Chen M, Lin Z, Ren Y, et al. Universal synthesis of rare earth-doped FeP nanorod arrays for the Hydrogen Evolution Reaction. Materials Chemistry Frontiers. 2023; 7(18): 4132–4141. doi: 10.1039/d3qm00516j
7. Oh JH, Lee YH, Kim M, et al. Evaluation of thermal plasma-synthesized cobalt boride nanoparticles as efficient water-splitting catalysts. Journal of Environmental Chemical Engineering. 2023; 11(2): 109578. doi: 10.1016/j.jece.2023.109578
8. Bao W, Lu K, Fu P, et al. Solution plasma-assisted synthesis of oxyhydroxides for advanced electrocatalytic water splitting. Chemical Engineering Journal. 2023; 474: 145826. doi: 10.1016/j.cej.2023.145826
9. Cui M, Wang F, Zhao W, et al. Plasma-synthesized platinum single atom and nanoparticle catalysts for high-current–density hydrogen evolution. Chemical Engineering Journal. 2023; 460: 141676. doi: 10.1016/j.cej.2023.141676
10. Yan P, Yang T, Lin M, et al. “One Stone Five Birds” Plasma Activation Strategy Synergistic with Ru Single Atoms Doping Boosting the Hydrogen Evolution Performance of Metal Hydroxide. Advanced Functional Materials. 2023; 33(25). doi: 10.1002/adfm.202301343
11. Shen Z, Yu Y, Zhao Z, et al. N, O trans-coordinating silver single-atom catalyst for robust and efficient ammonia electrosynthesis from nitrate. Applied Catalysis B: Environmental. 2023; 331: 122687. doi: 10.1016/j.apcatb.2023.122687
12. Qi Q, Shao D, Zhou Y, et al. Plasma-induced implanting of active species in metal–organic frameworks for efficient Hydrogen Evolution Reaction. Journal of Materials Chemistry A. 2023; 11(29): 15663–15669. doi: 10.1039/d3ta02610h
13. Wnukowski M. Methane Pyrolysis with the Use of Plasma: Review of Plasma Reactors and Process Products. Energies. 2023; 16(18): 6441. doi: 10.3390/en16186441
14. Alhamed H, Behar O, Saxena S, et al. From methane to hydrogen: A comprehensive review to assess the efficiency and potential of turquoise hydrogen technologies. International Journal of Hydrogen Energy. 2024; 68: 635–662. doi: 10.1016/j.ijhydene.2024.04.231
15. Ren Y, Li S, Yu C, et al. NH3 Electrosynthesis from N2 Molecules: Progresses, Challenges, and Future Perspectives. Journal of the American Chemical Society. 2024; 146(10): 6409–6421. doi: 10.1021/jacs.3c11676
16. Tian F, Zhou N, Chen W, et al. Progress in Green Ammonia Synthesis Technology: Catalytic Behavior of Ammonia Synthesis Catalysts. Advanced Sustainable Systems. 2024; 8(8). doi: 10.1002/adsu.202300618
17. Shahed Gharahshiran V, Zheng Y. Sustainable ammonia synthesis: An in-depth review of non-thermal plasma technologies. Journal of Energy Chemistry. 2024; 96: 1–38. doi: 10.1016/j.jechem.2024.04.018
18. Zhang Y, Niu J, Chen S, et al. Ammonia synthesis by nonthermal plasma catalysis: A review on recent research progress. Journal of Physics D: Applied Physics. 2024; 57(32): 323001. doi: 10.1088/1361-6463/ad4717
19. Li J, Xiong Q, Mu X, Li L. Recent Advances in Ammonia Synthesis: From Haber‐Bosch Process to External Field Driven Strategies. ChemSusChem. 2024; 17(15). doi: 10.1002/cssc.202301775
20. Panchal D, Lu Q, Sakaushi K, Zhang X. Advanced cold plasma-assisted technology for green and sustainable ammonia synthesis. Chemical Engineering Journal. 2024; 498: 154920. doi: 10.1016/j.cej.2024.154920
21. Zhang B, Li J, Zuo H, et al. Strategies for avoiding the scaling relationship in ammonia synthesis with non-thermal plasma methods—the “shift” or “break” approach. Green Chemistry. 2024; 26(7): 3670–3687. doi: 10.1039/d3gc05006h
22. Gorbanev Y, Fedirchyk I, Bogaerts A. Plasma catalysis in ammonia production and decomposition: Use it, or lose it? Current Opinion in Green and Sustainable Chemistry. 2024; 47: 100916. doi: 10.1016/j.cogsc.2024.100916
23. Bayer BN, Bhan A, Bruggeman PJ. Reaction Pathways and Energy Consumption in NH3 Decomposition for H2 Production by Low Temperature, Atmospheric Pressure Plasma. Plasma Chemistry and Plasma Processing. 2024; 44(6): 2101–2118. doi: 10.1007/s11090-024-10501-8
24. Zhang M, Chen Q, Zhou G, et al. Low-temperature chemistry in plasma-driven ammonia oxidative pyrolysis. Green Energy & Environment. 2024; 9(9): 1477–1488. doi: 10.1016/j.gee.2023.05.010
25. Zhang M, Chen Q, Liu N, et al. Kinetic insights into ammonia-hydrogen doped ignition and emission assisted by nanosecond pulsed discharge. International Journal of Hydrogen Energy. 2024; 78: 773–782. doi: 10.1016/j.ijhydene.2024.06.291
26. Urabe K, Toyoda M, Matsuoka Y, Eriguchi K. Investigation of small-fraction molecular impurities in high-pressure helium plasmas using optical plasma diagnostic methods. Plasma Sources Science and Technology. 2024; 33(2): 025011. doi: 10.1088/1361-6595/ad1f38
27. Vasilev M, Suiter J, Bohl D, Thagard SM. Caffeine degradation in a plasma-liquid reactor with the lateral liquid flow: Elucidating the effects of mass transport on contaminant removal. Chemical Engineering Journal. 2023; 473: 144833. doi: 10.1016/j.cej.2023.144833
28. Guo Y, Guo X, Xu S, Shi J. Estimation of total forces of jets on liquid interfaces using image processing methods. Journal of Physics D: Applied Physics. 2024; 57(24): 245206. doi: 10.1088/1361-6463/ad33f6
29. Ouali A, Sebih L, Herrmann A, et al. Propagation of nanosecond discharge in an air gap containing a water droplet: Modelling and comparison with time-resolved images. Journal of Physics D: Applied Physics. 2024; 57(31): 315202. doi: 10.1088/1361-6463/ad44a3
30. Frolov A, Stelmashuk V, Kolacek K, et al. Pressure in underwater spark discharge initiated with the help of bubble injection and its evaluation based on H-alpha line broadening. Journal of Physics D: Applied Physics. 2023; 56(28): 285201. doi: 10.1088/1361-6463/accaf3
31. Klose SJ, Ellis J, Riedel F, et al. The spatial distribution of hydrogen and oxygen atoms in a cold atmospheric pressure plasma jet. Plasma Sources Science and Technology. 2020; 29(12): 125018. doi: 10.1088/1361-6595/abcc4f
32. Rooij O van, Ahlborn O, Sobota A. Electron density in a non-thermal atmospheric discharge in contact with water and the effect of water temperature on plasma-water interactions. Journal of Physics D: Applied Physics. 2024; 57(38): 385206. doi: 10.1088/1361-6463/ad59b0
33. Čech J, Sťahel P, Prokeš L, et al. CaviPlasma: Parametric study of discharge parameters of high-throughput water plasma treatment technology in glow-like discharge regime. Plasma Sources Science and Technology. 2024; 33(11): 115005. doi: 10.1088/1361-6595/ad7e4e
34. Belmonte T, Bruggeman P. Optical Diagnostics of Discharges in and in Contact With Liquids. Plasma Processes and Polymers. 2024; 22(1). doi: 10.1002/ppap.202400213
35. Feng BW, Zhong XX, Zhang Q, et al. Effect of duty cycle on pulsed discharge atmospheric pressure plasma: Discharge volume and remnant electron density. Plasma Sources Science and Technology. 2020; 29(8): 085017. doi: 10.1088/1361-6595/aba772
36. Song Z, Fridman A, Dobrynin D. Effects of liquid properties on the development of nanosecond-pulsed plasma inside of liquid: Comparison of water and liquid nitrogen. Journal of Physics D: Applied Physics. 2024; 57(17): 175203. doi: 10.1088/1361-6463/ad211f
37. Zhu X, Zhang X, Tao Y, et al. Plasma synthesis of Pt/C catalysts and their electrocatalytic performance. Journal of Physics D: Applied Physics. 2024; 57(50): 505201. doi: 10.1088/1361-6463/ad7a7f
38. Liu X, Chen G, Guo Y, et al. Fabric-like rhodium-nickel-tungsten oxide nanosheets for highly-efficient electrocatalytic H2 generation in an alkaline electrolyte. Journal of Colloid and Interface Science. 2024; 659: 895–904. doi: 10.1016/j.jcis.2024.01.060
39. Zhang J, Nie W, Wang R, et al. Plasma technique regulates the electronic structure and dual functional catalytic performance of p-VNiCoPy/NiFeOx heterojunction catalysts for hydrogen production through overall water splitting. Journal of Alloys and Compounds. 2024; 1004: 175900. doi: 10.1016/j.jallcom.2024.175900
40. Wang J, Chen G, Zhang Y, et al. Plasma-assisted in-situ engineering carambola-like indium-doped Ni-Co selenides for robust hydrogen evolution. Applied Surface Science. 2023; 625: 157198. doi: 10.1016/j.apsusc.2023.157198
41. Liu F, Wang F, Sun X, et al. Plasma-induced vacancies in CoS2 electrocatalysts to activate sulfur sites for Hydrogen Evolution Reaction. International Journal of Hydrogen Energy. 2024; 58: 941–947. doi: 10.1016/j.ijhydene.2024.01.320
42. Ning Z, Li R, Xin K, et al. Elevating hydrogen evolution performance with plasma-induced sulfur vacancies and heteroatom doping in hollow-structured MnS–CoS catalysts. Journal of Alloys and Compounds. 2024; 977: 173461. doi: 10.1016/j.jallcom.2024.173461
43. Chen G, Xiang H, Guo Y, et al. Yttrium- and nitrogen-doped NiCo phosphide nanosheets for high-efficiency water electrolysis. Carbon Energy. 2024; 6(8): e522. doi: 10.1002/cey2.522
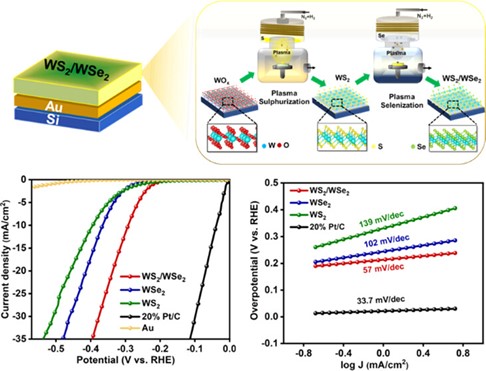
44. Rehman B, Kimbulapitiya KMMDK, Date M, et al. Rational Design of Phase-Engineered WS2/WSe2 Heterostructures by Low-Temperature Plasma-Assisted Sulfurization and Selenization toward Enhanced HER Performance. ACS Applied Materials & Interfaces. 2024; 16(25): 32490–32502. doi: 10.1021/acsami.4c03513
45. He W, Wu S, Zhang Z, et al. Modulating vacancies of graphene supported FeNi2S4 electrocatalysts by radio-frequency plasma for overall water splitting. Journal of Physics D: Applied Physics. 2024; 57(40): 405501. doi: 10.1088/1361-6463/ad5f39
46. Wei Y, Yi L, Zhang S, et al. Ni–Mo nitride synthesized via mild plasma for efficient alkaline hydrogen evolution electrocatalysis. Journal of Materials Chemistry A. 2024; 12(14): 8534–8542. doi: 10.1039/d3ta07566d
47. Zhao K, Zhang J, Li H, et al. Boosting HER performance by using plasma prepared N-doped CNTs to support Pt nanoparticles. International Journal of Hydrogen Energy. 2024; 90: 1271–1278. doi: 10.1016/j.ijhydene.2024.10.103
48. Park J, Cho I, Jeon H, et al. Conversion of Layered WS2 Crystals into Mixed‐Domain Electrochemical Catalysts by Plasma-Assisted Surface Reconstruction. Advanced Materials. 2024; 36(25). doi: 10.1002/adma.202314031
49. Park KH, Mathew S, Kim KH, et al. Plasma Treatment on Cobalt Sulfide Nanoparticle/Nickel Foam Electrocatalysts for Hydrogen Evolution Reaction. ACS Applied Nano Materials. 2024; 7(8): 8547–8556. doi: 10.1021/acsanm.3c05097
50. Ma X, Zhang X, Huang J, et al. In situ synthesis of self-supported Ir/IrO2 heterostructures via Ar-H2 plasma as efficient bifunctional catalyst for overall water splitting in acidic media. Applied Surface Science. 2024; 642: 158558. doi: 10.1016/j.apsusc.2023.158558
51. Pang N, Li Y, Wang C, et al. Facilitating the Hydrogen Evolution Reaction on Basal-Plane S Sites on MoS2@Ni3S2 by Dual Ti and N Plasma Treatment. ACS Applied Materials & Interfaces. 2024; 16(31): 40881–40893. doi: 10.1021/acsami.4c05758
52. Kulkarni R, Lingamdinne LP, Koduru JR, et al. Atmospheric oxygen plasma-activated novel multicomponent transition metal phosphides (MnCoCu–P) for enhanced electrocatalytic water splitting to green hydrogen production: A universal catalyst across various pH electrolytes. International Journal of Hydrogen Energy. 2024; 76: 341–352. doi: 10.1016/j.ijhydene.2024.05.352
53. Soo JZ, Riaz A, Zhang D, et al. Enhancing the Hydrogen Evolution Reaction Performance of Solution-Corroded NiMo via Plasma Modification. Chemistry of Materials. 2024; 36(9): 4164–4173. doi: 10.1021/acs.chemmater.3c02978
54. Sun H, Liu C, Ma Z, et al. A general strategy for synthesizing bimetallic Pt-based nanoclusters supported on carbon black via non-thermal plasma. International Journal of Hydrogen Energy. 2024; 83: 317–325. doi: 10.1016/j.ijhydene.2024.08.135
55. Liu R, Hao Y, Wang T, et al. Hybrid plasma-thermal system for methane conversion to ethylene and hydrogen. Chemical Engineering Journal. 2023; 463: 142442. doi: 10.1016/j.cej.2023.142442
56. Liu R, Morais E, Li D, et al. Hybrid plasma catalysis-thermal system for non-oxidative coupling of methane to ethylene and hydrogen. Chemical Engineering Journal. 2024; 498: 155733. doi: 10.1016/j.cej.2024.155733
57. Lian H, Sun Z, Ru Y, et al. Warm plasma catalytic coreforming of dilute bioethanol and methane for hydrogen production. Plasma Processes and Polymers. 2023; 21(1). doi: 10.1002/ppap.202300062
58. Mašláni A, Hlína M, Hrabovský M, et al. Impact of natural gas composition on steam thermal plasma assisted pyrolysis for hydrogen and solid carbon production. Energy Conversion and Management. 2023; 297: 117748. doi: 10.1016/j.enconman.2023.117748
59. Wang Q, Sun S, Yang Y, et al. Efficient conversion of methane in aqueous solution assisted by microwave plasma technology with a novel electrode. Energy. 2024; 289: 130023. doi: 10.1016/j.energy.2023.130023
60. Batukaev ТS, Bilera IV, Krashevskaya GV, et al. Hydrogen production in microwave discharge in water with barbotage of methane at atmospheric pressure: Experiment and modeling. Plasma Processes and Polymers. 2024; 22(3). doi: 10.1002/ppap.202400139
61. Maerivoet S, Wanten B, De Meyer R, et al. Effect of O2 on Plasma-Based Dry Reforming of Methane: Revealing the Optimal Gas Composition via Experiments and Modeling of an Atmospheric Pressure Glow Discharge. ACS Sustainable Chemistry & Engineering. 2024; 12(30): 11419–11434. doi: 10.1021/acssuschemeng.4c04283
62. Lee DH, Song YH, Kim KT, et al. Comparative Study of Methane Activation Process by Different Plasma Sources. Plasma Chemistry and Plasma Processing. 2013; 33(4): 647–661. doi: 10.1007/s11090-013-9456-6
63. Zhang H, Du C, Wu A, et al. Rotating gliding arc assisted methane decomposition in nitrogen for hydrogen production. International Journal of Hydrogen Energy. 2014; 39(24): 12620–12635. doi: 10.1016/j.ijhydene.2014.06.047
64. Hrabovsky M, Hlina M, Kopecky V, et al. Steam Plasma Methane Reforming for Hydrogen Production. Plasma Chemistry and Plasma Processing. 2018; 38(4): 743–758. doi: 10.1007/s11090-018-9891-5
65. Kim SS, Jorat M, Voecks G, et al. Hydrogen from steam methane reforming by catalytic nonthermal plasma using a dielectric barrier discharge reactor. AIChE Journal. 2019; 66(4). doi: 10.1002/aic.16880
66. Geng F, Haribal VP, Hicks JC. Non-thermal plasma-assisted steam methane reforming for electrically-driven hydrogen production. Applied Catalysis A: General. 2022; 647: 118903. doi: 10.1016/j.apcata.2022.118903
67. Popov SD, Subbotin DI, Popov VE, et al. Electric Arc Methods of Production Hydrogen from Hydrocarbons. High Energy Chemistry. 2023; 57(S1): S155–S158. doi: 10.1134/s0018143923070330
68. Fulcheri L, Rohani VJ, Wyse E, et al. An energy-efficient plasma methane pyrolysis process for high yields of carbon black and hydrogen. International Journal of Hydrogen Energy. 2023; 48(8): 2920–2928. doi: 10.1016/j.ijhydene.2022.10.144
69. Nguyen HM, Gorky F, Guthrie S, et al. Plasma catalytic non-oxidative methane conversion to hydrogen and value-added hydrocarbons on zeolite 13X. Energy Conversion and Management. 2023; 286: 117082. doi: 10.1016/j.enconman.2023.117082
70. Wang Q, Wang Y, Sun J, et al. Hydrogen production from simulated seawater by microwave liquid discharge: A new way of green production. Chemical Engineering Journal. 2023; 465: 142872. doi: 10.1016/j.cej.2023.142872
71. Bajpai A, Mehta S, Joshi K, et al. Hydrogen from catalytic non-thermal plasma-assisted steam methane reforming reaction. International Journal of Hydrogen Energy. 2023; 48(63): 24328–24341. doi: 10.1016/j.ijhydene.2023.03.281
72. Ali Z, Song H, Trieu Nguyen UN, et al. Hydrogen and Solid Carbon Production via Methane Pyrolysis in a Rotating Gliding Arc Plasma Reactor. ChemSusChem. 2024. doi: 10.1002/cssc.202401602
73. Garcia-Villalva R, Biset-Peiró M, Murcia-López S, et al. Synergies between Plasma and Thermal Catalysis on Steam Methane Reforming for Hydrogen Production. ACS Sustainable Chemistry & Engineering. 2024; 12(50): 18276–18286. doi: 10.1021/acssuschemeng.4c07998
74. Gang Y, Long Y, Wang K, et al. Plasma Catalytic Non-Oxidative Conversion of Methane into Hydrogen and Light Hydrocarbons. Plasma Chemistry and Plasma Processing. 2024; 44(6): 2011–2029. doi: 10.1007/s11090-024-10497-1
75. Wang S, Wang J, Feng D, et al. Plasma-induced methane catalytic cracking: Effects of experimental conditions. International Journal of Hydrogen Energy. 2024; 63: 284–293. doi: 10.1016/j.ijhydene.2024.03.178
76. He H, Wang C, Ma H, et al. Improving energy consumption in plasma reforming of methane through gliding arc. Energy Conversion and Management. 2025; 325: 119413. doi: 10.1016/j.enconman.2024.119413
77. Bilera IV, Lebedev YuA, Titov AYu, et al. Modeling of Acetylene Formation from Methane in a Plasma Jet. High Energy Chemistry. 2024; 58(3): 332–342. doi: 10.1134/s0018143924700127
78. Essiptchouk A, Miranda F, Petraconi G. Comparative analysis of methane conversion: Pyrolysis, dry and steam thermal plasma reforming. Journal of Physics D: Applied Physics. 2024; 57(24): 245201. doi: 10.1088/1361-6463/ad31e7
79. Morais E, Delikonstantis E, Scapinello M, et al. Methane coupling in nanosecond pulsed plasmas: Correlation between temperature and pressure and effects on product selectivity. Chemical Engineering Journal. 2023; 462: 142227. doi: 10.1016/j.cej.2023.142227
80. Morais E, Bogaerts A. Modelling the dynamics of hydrogen synthesis from methane in nanosecond-pulsed plasmas. Plasma Processes and Polymers. 2024; 21(1). doi: 10.1002/ppap.202300149
81. Yuan X, Sun J, Ma Y, et al. A kinetic study of nonthermal plasma pyrolysis of methane: Insights into hydrogen and carbon material production. Chemical Engineering Journal. 2024; 499: 156396. doi: 10.1016/j.cej.2024.156396
82. Zhao Z, Qi Y, Cai K. Research on the combustion mechanism of plasma-induced ammonia-hydrogen jet ignition engine. International Journal of Hydrogen Energy. 2024; 65: 398–409. doi: 10.1016/j.ijhydene.2024.04.047
83. Zhan Q, Ban Y, Zhang F, et al. Numerical simulation of flame propagation characteristics of NH3/Air flames assisted by non-equilibrium plasma discharge. Combustion and Flame. 2025; 271: 113809. doi: 10.1016/j.combustflame.2024.113809
84. Yin SF, Xu BQ, Ng CF, et al. Nano Ru/CNTs: A highly active and stable catalyst for the generation of COx-free hydrogen in ammonia decomposition. Applied Catalysis B: Environmental. 2004; 48(4): 237–241. doi: 10.1016/j.apcatb.2003.10.013
85. Chen C, Wu K, Ren H, et al. Ru-Based Catalysts for Ammonia Decomposition: A Mini-Review. Energy & Fuels. 2021; 35(15): 11693–11706. doi: 10.1021/acs.energyfuels.1c01261
86. Fedirchyk I, Tsonev I, Quiroz Marnef R, et al. Plasma-assisted NH3 cracking in warm plasma reactors for green H2 production. Chemical Engineering Journal. 2024; 499: 155946. doi: 10.1016/j.cej.2024.155946
87. Akiyama M, Aihara K, Sawaguchi T, et al. Ammonia decomposition to clean hydrogen using non-thermal atmospheric-pressure plasma. International Journal of Hydrogen Energy. 2018; 43(31): 14493–14497. doi: 10.1016/j.ijhydene.2018.06.022
88. Andersen JA, Christensen JM, Østberg M, et al. Plasma-catalytic ammonia decomposition using a packed-bed dielectric barrier discharge reactor. International Journal of Hydrogen Energy. 2022; 47(75): 32081–32091. doi: 10.1016/j.ijhydene.2022.07.102
89. Wang Z, He G, Zhang H, et al. Plasma-Promoted Ammonia Decomposition over Supported Ruthenium Catalysts for Cox-Free H2 Production. ChemSusChem. 2023; 16(24). doi: 10.1002/cssc.202202370
90. Lin QF, Jiang YM, Liu CZ, et al. Instantaneous hydrogen production from ammonia by non-thermal arc plasma combining with catalyst. Energy Reports. 2021; 7: 4064–4070. doi: 10.1016/j.egyr.2021.06.087
91. Młotek M, Perron M, Krawczyk K. Ammonia Decomposition in a Gliding Discharge Plasma. Energy Technology. 2021; 9(12). doi: 10.1002/ente.202100677
92. Zhang X, Cha MS. Ammonia cracking for hydrogen production using a microwave argon plasma jet. Journal of Physics D: Applied Physics. 2023; 57(6): 065203. doi: 10.1088/1361-6463/ad0988
93. Zhang X, Cha MS. Optimizing ammonia cracking in microwave argon plasma: Temperature control and ammonia delivery. Chemical Engineering Journal. 2024; 496: 154289. doi: 10.1016/j.cej.2024.154289
94. Bang S, Snoeckx R, Cha MS. Kinetic Study for Plasma Assisted Cracking of NH3: Approaches and Challenges. The Journal of Physical Chemistry A. 2023; 127(5): 1271–1282. doi: 10.1021/acs.jpca.2c06919
95. Andersen JA, van ’t Veer K, Christensen JM, et al. Ammonia decomposition in a dielectric barrier discharge plasma: Insights from experiments and kinetic modeling. Chemical Engineering Science. 2023; 271: 118550. doi: 10.1016/j.ces.2023.118550
96. Hayakawa Y, Kambara S, Miura T. Hydrogen production from ammonia by the plasma membrane reactor. International Journal of Hydrogen Energy. 2020; 45(56): 32082–32088. doi: 10.1016/j.ijhydene.2020.08.178
97. Navascués P, Obrero-Pérez JM, Cotrino J, et al. Unraveling Discharge and Surface Mechanisms in Plasma-Assisted Ammonia Reactions. ACS Sustainable Chemistry & Engineering. 2020; 8(39): 14855–14866. doi: 10.1021/acssuschemeng.0c04461
98. Gorky F, Lucero JM, Crawford JM, et al. Insights on cold plasma ammonia synthesis and decomposition using alkaline earth metal-based perovskites. Catalysis Science & Technology. 2021; 11(15): 5109–5118. doi: 10.1039/d1cy00729g
99. Zhang S, Zhao Y, Shi R, et al. Photocatalytic ammonia synthesis: Recent progress and future. EnergyChem. 2019; 1(2): 100013. doi: 10.1016/j.enchem.2019.100013
100. Sadiek I, Fleisher AJ, Hayden J, et al. Dual-comb spectroscopy of ammonia formation in non-thermal plasmas. Communications Chemistry. 2024; 7(1): 110. doi: 10.1038/s42004-024-01190-7
101. Van Duc Long N, Pourali N, Lamichhane P, et al. Catalytic Ammonia Formation in a Microreaction Chamber with Electrically Intensified Arc Plasma. ChemCatChem. 2024; 16(13). doi: 10.1002/cctc.202400005
102. Li K, Chen S, Li M, et al. Plasma-catalyzed ammonia synthesis over La(OH)3 catalyst: Effects of basic sites, oxygen vacancies, and H2 plasma treatment. International Journal of Hydrogen Energy. 2024; 59: 1287–1296. doi: 10.1016/j.ijhydene.2024.02.123
103. Veng V, Ibrahim SA, Tabu B, et al. Ammonia Synthesis via Membrane Dielectric-Barrier Discharge Reactor Integrated with Metal Catalyst. Plasma Chemistry and Plasma Processing. 2024; 44(6): 2031–2055. doi: 10.1007/s11090-024-10502-7
104. Zhang B, Li J, Zuo H, et al. Reinforcement of fluidized catalysts with DBD plasma assisted for green ammonia synthesis. International Journal of Hydrogen Energy. 2024; 67: 521–531. doi: 10.1016/j.ijhydene.2024.04.079
105. Horiuchi Y, Kamei G, Saito M, et al. Development of Ruthenium-loaded Alkaline-earth Titanates as Catalysts for Ammonia Synthesis. Chemistry Letters. 2013; 42(10): 1282–1284. doi: 10.1246/cl.130574
106. Kim H, Teramoto Y, Ogata A, et al. Atmospheric-pressure nonthermal plasma synthesis of ammonia over ruthenium catalysts. Plasma Processes and Polymers. 2017; 14(6). doi: 10.1002/ppap.201600157
107. Wang Y, Craven M, Yu X, et al. Plasma-Enhanced Catalytic Synthesis of Ammonia over a Ni/Al2O3Catalyst at Near-Room Temperature: Insights into the Importance of the Catalyst Surface on the Reaction Mechanism. ACS Catalysis. 2019; 9(12): 10780–10793. doi: 10.1021/acscatal.9b02538
108. Shah J, Wu T, Lucero J, et al. Nonthermal Plasma Synthesis of Ammonia over Ni-MOF-74. ACS Sustainable Chemistry & Engineering. 2019; 7(1): 377–383. doi: 10.1021/acssuschemeng.8b03705
109. Liu Y, Wang CW, Xu XF, et al. Synergistic Effect of Co–Ni Bimetal on Plasma Catalytic Ammonia Synthesis. Plasma Chemistry and Plasma Processing. 2022; 42(2): 267–282. doi: 10.1007/s11090-021-10223-1
110. Li K, Chen S, Wang H, et al. Plasma-assisted ammonia synthesis over Ni/LaOF: Dual active centers consisting of oxygen vacancies and Ni. Applied Catalysis A: General. 2023; 650: 118983. doi: 10.1016/j.apcata.2022.118983
111. Wang Y, Yang W, Xu S, et al. Shielding Protection by Mesoporous Catalysts for Improving Plasma-Catalytic Ambient Ammonia Synthesis. Journal of the American Chemical Society. 2022; 144(27): 12020–12031. doi: 10.1021/jacs.2c01950
112. Nguyen HM, Gorky F, Guthrie S, Carreon ML. Sustainable ammonia synthesis from nitrogen wet with sea water by single-step plasma catalysis. Catalysis Today. 2023; 418: 114141. doi: 10.1016/j.cattod.2023.114141
113. Liu Y, Xu X, Song Q, et al. Co-Ni/MOF-74 catalyst packed-bed DBD plasma for ammonia synthesis. Plasma Processes and Polymers. 2024; 21(2). doi: 10.1002/ppap.202300086
114. Zen S, Takeuchi N, Teramoto Y. Ammonia synthesis using atmospheric pressure fluidized bed plasma. Journal of Physics D: Applied Physics. 2023; 57(11): 115203. doi: 10.1088/1361-6463/ad144b
115. Bajpai A, Kumar S. Tailoring the surface acidity of catalyst to enhance nonthermal plasma-assisted ammonia synthesis rates. Molecular Catalysis. 2024; 557: 113961. doi: 10.1016/j.mcat.2024.113961
116. Jing Y, Gong F, Wang S, et al. Activating the synergistic effect in Ni-Co bimetallic MOF for enhanced plasma-assisted ammonia synthesis. Fuel. 2024; 368: 131686. doi: 10.1016/j.fuel.2024.131686
117. Zhou G, Wang Z, Wang X, et al. Nonthermal-Plasma-Catalytic Ammonia Synthesis Using Fe2O3/CeO2 Mechanically Mixed with Al2O3: Insights into the Promoting Effect of Plasma Discharge Enhancement on the Role of Catalysts. ACS Sustainable Chemistry & Engineering. 2024; 12(38): 14349–14362. doi: 10.1021/acssuschemeng.4c06283
118. Chen J, Tang T, Wu X, et al. Unlocking Efficient Synergistic Plasma-Catalyst Ammonia Synthesis: System Optimization and Catalyst Support Screening. Energy & Fuels. 2024; 38(11): 10345–10356. doi: 10.1021/acs.energyfuels.4c00702
119. Song Q, Yin X, Zhang H. Ni-MOF-74 Derived Carbon-Based Ni Catalysts for Efficient Catalytic Ammonia Synthesis via Pulsed DBD Plasma. Plasma Processes and Polymers. 2024; 22(3). doi: 10.1002/ppap.202400173
120. Xu X, Sun M, Song Q, et al. Dielectric barrier discharge plasma-assisted catalytic ammonia synthesis: Synergistic effect of Ni-MOF-74 catalyst and nanosecond pulsed plasma. Plasma Science and Technology. 2024; 26(6): 064005. doi: 10.1088/2058-6272/ad1fd8
121. Lu K, Xu Y, Yuan H, et al. Non-thermal plasma synergistic Ni/Al2O3 for ammonia synthesis: Configuration and optimization of a double dielectric barrier discharge reactor. International Journal of Hydrogen Energy. 2025; 97: 835–844. doi: 10.1016/j.ijhydene.2024.11.462
122. Hippler R, Cada M, Knizek A, et al. Generation of Ammonia in a Pulsed Hollow Cathode Discharge Operated in an Ar/H2/N2 Gas Mixture Detected by Fourier Transform Infrared. ACS Sustainable Chemistry & Engineering. 2024; 12(48): 17443–17449. doi: 10.1021/acssuschemeng.4c08054
123. Liu N, Mao X, Kondratowicz C, et al. Unraveling Nonequilibrium Generation of Atomic Nitrogen and Hydrogen in Plasma-Aided Ammonia Synthesis. ACS Energy Letters. 2024; 9(5): 2031–2036. doi: 10.1021/acsenergylett.4c00729
124. Lele AD, Xu Y, Ju Y. Modelling the effect of surface charging on plasma synthesis of ammonia using DFT. Physical Chemistry Chemical Physics. 2024; 26(12): 9453–9461. doi: 10.1039/d3cp06050k
125. Lin Z, Abe S, Chen Z, et al. Kinetic Modeling Analysis of Ar Addition to Atmospheric Pressure N2–H2 Plasma for Plasma-Assisted Catalytic Synthesis of NH3. The Journal of Physical Chemistry A. 2024; 128(12). doi: 10.1021/acs.jpca.3c06841
126. Vervloedt SCL, von Keudell A. Ammonia synthesis by plasma catalysis in an atmospheric RF helium plasma. Plasma Sources Science and Technology. 2024; 33(4): 045005. doi: 10.1088/1361-6595/ad38d6
127. Ramoy M, Shirai N, Sasaki K. Catalyst-free synthesis of ammonia using dc-driven atmospheric-pressure plasma in contact with water. Journal of Physics D: Applied Physics. 2023; 57(1): 01LT01. doi: 10.1088/1361-6463/acfdb7
128. Xu X, Sun M, Song Q, et al. Nanosecond pulsed gliding arc plasma for ammonia synthesis: Better insight from discharge mode and vibrational temperature. Journal of Physics D: Applied Physics. 2024; 57(41): 415206. doi: 10.1088/1361-6463/ad5f3d
129. Maeng J, Jang D, Ha J, et al. Oxygen Vacancy-Controlled CuOx/N,Se Co-Doped Porous Carbon via Plasma-Treatment for Enhanced Electro-Reduction of Nitrate to Green Ammonia. Small. 2024; 20(37). doi: 10.1002/smll.202403253
130. Hu S, Lv B, Xu X, et al. Rapid plasma preparation of CuO nanowires for efficient ammonia synthesis. Surfaces and Interfaces. 2024; 48: 104286. doi: 10.1016/j.surfin.2024.104286
131. Zhang L, Guo X, Zhang S, et al. Hybrid Double Atom Catalysts for Hydrogen Evolution Reaction: A Sweet Marriage of Metal and Nonmetal. Advanced Energy Materials. 2023; 14(2). doi: 10.1002/aenm.202302754
132. Sun H, Li Y, Gao L, et al. High throughput screening of single atomic catalysts with optimized local structures for the electrochemical oxygen reduction by machine learning. Journal of Energy Chemistry. 2023; 81: 349–357. doi: 10.1016/j.jechem.2023.02.045




