制氢技术简述
DOI:
https://doi.org/10.18686/cncest.v2i1.145关键词:
氢能源;新能源利用;制氢技术;再生能源摘要
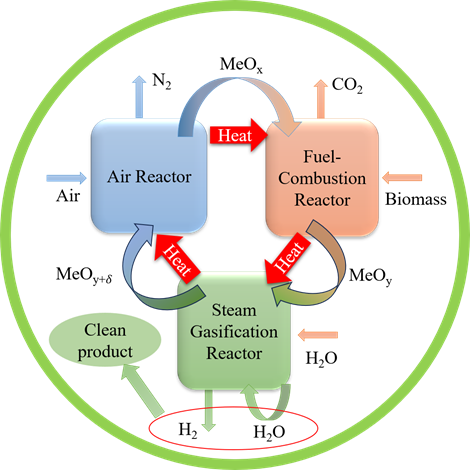
由于使用化石燃料产生了一系列问题,因此有必要开发和优化替代能源技术。尽管氢是一种理想的能源形式,但其主要来源仍然是通过传统方法获得的化石燃料。因此,人们对多种制氢资源和技术进行了研究,为清洁有效地制氢提供了可行性。本文对制氢技术进行了小型综述,包括可再生能源、化学循环、水电解、光催化和等离子体。
##submission.downloads##
已出版
文章引用
期
栏目
执照
版权声明
##submission.license.cc.by4.footer##参考
1. Cui P, Yao D, Ma Z, et al. Life cycle water footprint comparison of biomass-to-hydrogen and coal-to-hydrogen processes. Science of The Total Environment. 2021; 773: 145056. doi: 10.1016/j.scitotenv.2021.145056
2. Liu W, Liu C, Gogoi P, et al. Overview of Biomass Conversion to Electricity and Hydrogen and Recent Developments in Low-Temperature Electrochemical Approaches. Engineering. 2020; 6(12): 1351-1363. doi: 10.1016/j.eng.2020.02.021
3. Ahmadi MH, Jashnani H, Chau KW, et al. Carbon dioxide emissions prediction of five Middle Eastern countries using artificial neural networks. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. 2019; 45(3): 9513-9525. doi: 10.1080/15567036.2019.1679914
4. Davis SJ, Lewis NS, Shaner M, et al. Net-zero emissions energy systems. Science. 2018; 360(6396). doi: 10.1126/science.aas9793
5. Boyd PG, Chidambaram A, García-Díez E, et al. Data-driven design of metal–organic frameworks for wet flue gas CO2 capture. Nature. 2019; 576(7786): 253-256. doi: 10.1038/s41586-019-1798-7
6. Kumar R, Ahmadi MH, Rajak DK, et al. A study on CO2 absorption using hybrid solvents in packed columns. International Journal of Low-Carbon Technologies. Published online October 14, 2019. doi: 10.1093/ijlct/ctz051
7. Roh HS. Nanocatalysts for Hydrogen Production. Catalysts. 2021; 11(2): 288. doi: 10.3390/catal11020288
8. Xu X, Zhou Q, Yu D. The future of hydrogen energy: Bio-hydrogen production technology. International Journal of Hydrogen Energy. 2022; 47(79): 33677-33698. doi: 10.1016/j.ijhydene.2022.07.261
9. Dawood F, Anda M, Shafiullah GM. Hydrogen production for energy: An overview. International Journal of Hydrogen Energy. 2020; 45(7): 3847-3869. doi: 10.1016/j.ijhydene.2019.12.059
10. Midilli A, Ay M, Dincer I, et al. On hydrogen and hydrogen energy strategies. Renewable and Sustainable Energy Reviews. 2005; 9(3): 255-271. doi: 10.1016/j.rser.2004.05.003
11. Tadlock T, Rubio O, Ristanovic D. The Green Hydrogen Revolution - Integrating Hydrogen Into Industrial Applications. 2022 IEEE IAS Petroleum and Chemical Industry Technical Conference (PCIC). Published online September 26, 2022. doi: 10.1109/pcic42668.2022.10181257
12. e Silva MPG da C, Miranda JC de C. Exergy efficiency of thermochemical syngas-to-ethanol production plants. SN Applied Sciences. 2021; 3(5). doi: 10.1007/s42452-021-04526-3
13. Gao Y, Jausseme C, Huang Z, et al. Hydrogen-Powered Aircraft: Hydrogen–electric hybrid propulsion for aviation. IEEE Electrification Magazine. 2022; 10(2): 17-26. doi: 10.1109/mele.2022.3165725
14. Huang J, Balcombe P, Feng Z. Technical and economic analysis of different colours of producing hydrogen in China. Fuel. 2023; 337: 127227. doi: 10.1016/j.fuel.2022.127227
15. Jang D, Kim K, Kim KH, et al. Techno-economic analysis and Monte Carlo simulation for green hydrogen production using offshore wind power plant. Energy Conversion and Management. 2022; 263: 115695. doi: 10.1016/j.enconman.2022.115695
16. Karayel GK, Javani N, Dincer I. Hydropower for green hydrogen production in Turkey. International Journal of Hydrogen Energy. 2023; 48(60): 22806-22817. doi: 10.1016/j.ijhydene.2022.04.084
17. Shiva Kumar S, Lim H. An overview of water electrolysis technologies for green hydrogen production. Energy Reports. 2022; 8: 13793-13813. doi: 10.1016/j.egyr.2022.10.127
18. Mazzeo D, Herdem MS, Matera N, et al. Green hydrogen production: Analysis for different single or combined large-scale photovoltaic and wind renewable systems. Renewable Energy. 2022; 200: 360-378. doi: 10.1016/j.renene.2022.09.057
19. Li Z, Zhang W, Zhang R, et al. Development of renewable energy multi-energy complementary hydrogen energy system (A Case Study in China): A review. Energy Exploration & Exploitation. 2020; 38(6): 2099-2127. doi: 10.1177/0144598720953512
20. Song HG, Chun YN. Tar decomposition-reforming conversion on microwave-heating carbon receptor. Energy. 2020; 199: 117482. doi: 10.1016/j.energy.2020.117482
21. Li Q, Wang Q, Kayamori A, et al. Experimental study and modeling of heavy tar steam reforming. Fuel Processing Technology. 2018; 178: 180-188. doi: 10.1016/j.fuproc.2018.05.020
22. Tan RS, Tuan Abdullah TA, Johari A, et al. Catalytic steam reforming of tar for enhancing hydrogen production from biomass gasification: a review. Frontiers in Energy. 2020; 14(3): 545-569. doi: 10.1007/s11708-020-0800-2
23. Yang H, Yan R, Chen H, et al. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel. 2007; 86(12-13): 1781-1788. doi: 10.1016/j.fuel.2006.12.013
24. Quan C, Gao N, Song Q. Pyrolysis of biomass components in a TGA and a fixed-bed reactor: Thermochemical behaviors, kinetics, and product characterization. Journal of Analytical and Applied Pyrolysis. 2016; 121: 84-92. doi: 10.1016/j.jaap.2016.07.005
25. Yang J, Rizkiana J, Widayatno WB, et al. Fast co-pyrolysis of low density polyethylene and biomass residue for oil production. Energy Conversion and Management. 2016; 120: 422-429. doi: 10.1016/j.enconman.2016.05.008
26. Chen YH, Schmid M, Kertthong T, et al. Reforming of toluene as a tar model compound over straw char containing fly ash. Biomass and Bioenergy. 2020; 141: 105657. doi: 10.1016/j.biombioe.2020.105657
27. Trinh QT, Nguyen AV, Huynh DC, et al. Mechanistic insights into the catalytic elimination of tar and the promotional effect of boron on it: first-principles study using toluene as a model compound. Catalysis Science & Technology. 2016; 6(15): 5871-5883. doi: 10.1039/c6cy00358c
28. Mukai D, Murai Y, Higo T, et al. In situ IR study for elucidating reaction mechanism of toluene steam reforming over Ni/La0.7Sr0.3AlO3−δ catalyst. Applied Catalysis A: General. 2013; 466: 190-197. doi: 10.1016/j.apcata.2013.06.052
29. Xie W, Yang J, Wang Q, et al. Layered perovskite-like La2−xCaxNiO4±δ derived catalysts for hydrogen production via auto-thermal reforming of acetic acid. Catalysis Science & Technology. 2018; 8(12): 3015-3024. doi: 10.1039/c8cy00116b
30. Goicoechea S, Ehrich H, Arias PL, et al. Thermodynamic analysis of acetic acid steam reforming for hydrogen production. Journal of Power Sources. 2015; 279: 312-322. doi: 10.1016/j.jpowsour.2015.01.012
31. Hoang TMC, Geerdink B, Sturm JM, et al. Steam reforming of acetic acid – A major component in the volatiles formed during gasification of humin. Applied Catalysis B: Environmental. 2015; 163: 74-82. doi: 10.1016/j.apcatb.2014.07.046
32. Wang S, Li X, Zhang F, et al. Bio-oil catalytic reforming without steam addition: Application to hydrogen production and studies on its mechanism. International Journal of Hydrogen Energy. 2013; 38(36): 16038-16047. doi: 10.1016/j.ijhydene.2013.10.032
33. Lewis WK, Gilliland ER, Reed WA. Reaction of Methane with Copper Oxide in a Fluidized Bed. Industrial & Engineering Chemistry. 1949; 41(6): 1227-1237. doi: 10.1021/ie50474a018
34. Güleç F, Okolie JA. Decarbonising bioenergy through biomass utilisation in chemical looping combustion and gasification: a review. Environmental Chemistry Letters. 2023; 22(1): 121-147. doi: 10.1007/s10311-023-01656-5
35. Das S, Biswas A, Tiwary CS, et al. Hydrogen production using chemical looping technology: A review with emphasis on H2 yield of various oxygen carriers. International Journal of Hydrogen Energy. 2022; 47(66): 28322-28352. doi: 10.1016/j.ijhydene.2022.06.170
36. Kang KS, Kim CH, Bae KK, et al. Oxygen-carrier selection and thermal analysis of the chemical-looping process for hydrogen production. International Journal of Hydrogen Energy. 2010; 35(22): 12246-12254. doi: 10.1016/j.ijhydene.2010.08.043
37. Kobayashi N, Itaya Y. Performance of iron oxide-based oxygen carrier in biomass pyrolysis. Journal of Chemical Engineering of Japan. 2018; 51(5): 469-475. doi: 10.1252/jcej.17we111
38. Chang Y, Li G, Ma S, et al. Effect of hierarchical pore structure of oxygen carrier on the performance of biomass chemical looping hydrogen generation. Energy. 2022; 254: 124301. doi: 10.1016/j.energy.2022.124301
39. Zeng DW, Peng S, Chen C, et al. Nanostructured Fe2O3/MgAl2O4 material prepared by colloidal crystal templated sol–gel method for chemical looping with hydrogen storage. International Journal of Hydrogen Energy. 2016; 41(48): 22711-22721. doi: 10.1016/j.ijhydene.2016.09.180
40. Liu YC, Ku Y, Tseng YH, et al. Fabrication of Fe2O3/TiO2 Oxygen Carriers for Chemical Looping Combustion and Hydrogen Generation. Aerosol and Air Quality Research. 2016; 16(8): 2023-2032. doi: 10.4209/aaqr.2015.10.0603
41. Shimokawa K, Atsumi T, Harada M, et al. Zinc-based spinel cathode materials for magnesium rechargeable batteries: toward the reversible spinel–rocksalt transition. Journal of Materials Chemistry A. 2019; 7(19): 12225-12235. doi: 10.1039/c9ta02281c
42. Hu Q, Shen Y, Chew JW, et al. Chemical looping gasification of biomass with Fe2O3/CaO as the oxygen carrier for hydrogen-enriched syngas production. Chemical Engineering Journal. 2020; 379: 122346. doi: 10.1016/j.cej.2019.122346
43. Liu C, Luo J, Dong H, et al. Hydrogen-rich syngas production form biomass char by chemical looping gasification with Fe/Ca-based oxygen carrier. Separation and Purification Technology. 2022; 300: 121912. doi: 10.1016/j.seppur.2022.121912
44. Huang Z, Deng Z, Chen D, He F, Li H. Performance of NiFe2O4 oxygen carrier in hydrogen production by chemical looping steam reforming. Modern Chemical Industry. 2018; 38(9): 90-95. doi: 10.16606/j.cnki.issn0253-4320.2018.09.021
45. Gao J, Pu G, Wang P, et al. Study on the reaction performance of Ce‐doped NiFe2O4 oxygen carriers in the process of chemical looping hydrogen production. International Journal of Energy Research. 2021; 46(3): 2810-2825. doi: 10.1002/er.7346
46. Wang Y, Zhu Q, Xie T, et al. Promoted Alkaline Hydrogen Evolution Reaction Performance of Ru/C by Introducing TiO2 Nanoparticles. ChemElectroChem. 2020; 7(5): 1182-1186. doi: 10.1002/celc.201902170
47. Guo JX, Yan DY, Qiu KW, et al. High electrocatalytic hydrogen evolution activity on a coupled Ru and CoO hybrid electrocatalyst. Journal of Energy Chemistry. 2019; 37: 143-147. doi: 10.1016/j.jechem.2018.12.011
48. Li R, Kuang P, Wageh S, et al. Potential-dependent reconstruction of Ni-based cuboid arrays for highly efficient hydrogen evolution coupled with electro-oxidation of organic compound. Chemical Engineering Journal. 2023; 453: 139797. doi: 10.1016/j.cej.2022.139797
49. Li J, Xu P, Zhou R, et al. Co9S8–Ni3S2 heterointerfaced nanotubes on Ni foam as highly efficient and flexible bifunctional electrodes for water splitting. Electrochimica Acta. 2019; 299: 152-162. doi: 10.1016/j.electacta.2019.01.001
50. An L, Wei C, Lu M, et al. Recent Development of Oxygen Evolution Electrocatalysts in Acidic Environment. Advanced Materials. 2021; 33(20). doi: 10.1002/adma.202006328
51. Fukazawa A, Tanaka K, Hashimoto Y, et al. Electrocatalytic asymmetric hydrogenation of α,β-unsaturated acids in a PEM reactor with cinchona-modified palladium catalysts. Electrochemistry Communications. 2020; 115: 106734. doi: 10.1016/j.elecom.2020.106734
52. Vincent I, Bessarabov D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renewable and Sustainable Energy Reviews. 2018; 81: 1690-1704. doi: 10.1016/j.rser.2017.05.258
53. Li C, Baek JB. The promise of hydrogen production from alkaline anion exchange membrane electrolyzers. Nano Energy. 2021; 87: 106162. doi: 10.1016/j.nanoen.2021.106162
54. Ham K, Hong S, Kang S, et al. Extensive Active-Site Formation in Trirutile CoSb2O6 by Oxygen Vacancy for Oxygen Evolution Reaction in Anion Exchange Membrane Water Splitting. ACS Energy Letters. 2021; 6(2): 364-370. doi: 10.1021/acsenergylett.0c02359
55. Lim A, Kim HJ, Henkensmeier D, et al. A study on electrode fabrication and operation variables affecting the performance of anion exchange membrane water electrolysis. Journal of Industrial and Engineering Chemistry. 2019; 76: 410-418. doi: 10.1016/j.jiec.2019.04.007
56. Varcoe JR, Atanassov P, Dekel DR, et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ Sci. 2014; 7(10): 3135-3191. doi: 10.1039/c4ee01303d
57. Mandal M. Recent Advancement on Anion Exchange Membranes for Fuel Cell and Water Electrolysis. ChemElectroChem. 2020; 8(1): 36-45. doi: 10.1002/celc.202001329
58. Jiang Z, Yi G, Yao X, et al. Durable and highly-efficient anion exchange membrane water electrolysis using poly(biphenyl alkylene) membrane. Chemical Engineering Journal. 2023; 467: 143442. doi: 10.1016/j.cej.2023.143442
59. Nechache A, Hody S. Alternative and innovative solid oxide electrolysis cell materials: A short review. Renewable and Sustainable Energy Reviews. 2021; 149: 111322. doi: 10.1016/j.rser.2021.111322
60. Zheng Y, Chen Z, Zhang J. Solid Oxide Electrolysis of H2O and CO2 to Produce Hydrogen and Low-Carbon Fuels. Electrochemical Energy Reviews. 2021; 4(3): 508-517. doi: 10.1007/s41918-021-00097-4
61. Liu RT, Xu ZL, Li FM, et al. Recent advances in proton exchange membrane water electrolysis. Chemical Society Reviews. 2023; 52(16): 5652-5683. doi: 10.1039/d2cs00681b
62. Cao J, Zhang W, Li Y, et al. Current status of hydrogen production in China. Progress in Chemistry. 2021; 33(12): 2215-2244. doi: 10.7536/PC201128
63. Tonks L, Langmuir I. A General Theory of the Plasma of an Arc. Physical Review. 1929; 34(6): 876-922. doi: 10.1103/physrev.34.876
64. Liu L, Zhang Z, Das S, et al. LaNiO3 as a precursor of Ni/La2O3 for reverse water-gas shift in DBD plasma: Effect of calcination temperature. Energy Conversion and Management. 2020; 206: 112475. doi: 10.1016/j.enconman.2020.112475
65. Chung KH, Park YK, Kim SJ, et al. CO2-free hydrogen production by liquid-phase plasma cracking from benzene over perovskite catalysts. International Journal of Hydrogen Energy. 2024; 52: 885-893. doi: 10.1016/j.ijhydene.2022.05.008
66. Alharthi FA, Ababtain AS, Alanazi HS, et al. Synthesis of Zn3V2O8/rGO Nanocomposite for Photocatalytic Hydrogen Production. Inorganics. 2023; 11(3): 93. doi: 10.3390/inorganics11030093
67. Zhang S, Liu D, Song L, et al. Significant improvement of TiO2 photocatalytic hydrogen generation by photothermic synergistic action and underlying mechanism. International Journal of Hydrogen Energy. 2023; 48(69): 26665-26675. doi: 10.1016/j.ijhydene.2023.03.279
68. He B, Xiao P, Wan S, et al. Rapid charge transfer endowed by interfacial Ni-O bonding in s-scheme heterojunction for efficient photocatalytic H2 and imine production. Angewandte Chemie-International Edition. 2023; 62(50): e202313172. doi: 10.1002/anie.202313172
69. Li J, Huang Z, Wang C, et al. Linkage effect in the bandgap-broken V2O5-GdCrO3 heterojunction by carbon allotropes for boosting photocatalytic H2 production. Applied Catalysis B: Environmental. 2024; 340: 123181. doi: 10.1016/j.apcatb.2023.123181
70. Li J, Wang X, Fang H, et al. Unraveling the role of surface and interfacial defects in hydrogen production to construct an all-in-one broken-gap photocatalyst. Journal of Materials Chemistry A. 2023; 11(46): 25639-25649. doi: 10.1039/d3ta03079b
71. Chelvam K, Hanafiah MM, Woon KS, et al. A review on the environmental performance of various hydrogen production technologies: An approach towards hydrogen economy. Energy Reports. 2024; 11: 369-383. doi: 10.1016/j.egyr.2023.11.060
72. Das A, Peu SD. A Comprehensive Review on Recent Advancements in Thermochemical Processes for Clean Hydrogen Production to Decarbonize the Energy Sector. Sustainability. 2022; 14(18): 11206. doi: 10.3390/su141811206
73. Demirbas A. Comparison of thermochemical conversion processes of biomass to hydrogen-rich gas mixtures. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. 2016; 38(20): 2971-2976. doi: 10.1080/15567036.2015.1122686
74. Preethi V, Kanmani S. Photocatalytic hydrogen production. Materials Science in Semiconductor Processing. 2013; 16(3): 561-575. doi: 10.1016/j.mssp.2013.02.001
75. Wang L, Wang S, Zhou J, et al. A scientometric review: Biomass gasification study from 2006 to 2020. ACS Omega. 2022; 7(43): 38246-38253. doi: 10.1021/acsomega.2c05527
76. Yan L, He B, Pei X, et al. Design and comparisons of three biomass based hydrogen generation systems with chemical looping process. International Journal of Hydrogen Energy. 2014; 39(31): 17540-17553. doi: 10.1016/j.ijhydene.2014.08.115




